4-Chloro-3,5-dimethylphenol
Synonym(s):4-Chloro-3,5-dimethylphenol;4-Chloro-3,5-xylenol;4-Chloro-sym-m-xylenol;p-Chloro-m-xylenol;PCMX
- CAS NO.:88-04-0
- Empirical Formula: C8H9ClO
- Molecular Weight: 156.61
- MDL number: MFCD00002324
- EINECS: 201-793-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
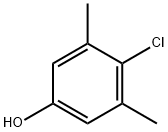
What is 4-Chloro-3,5-dimethylphenol?
Chemical properties
4-Chloro-3,5-dimethylphenol is white or cream crystalline powder with a characteristic odour
Chemical properties
White or cream-colored crystals or crystalline powder with a characteristic phenolic odor. Volatile in steam.
Originator
Septiderm ,Fougera ,US,1960
The Uses of 4-Chloro-3,5-dimethylphenol
4-Chloro-3,5-dimethylphenol is an antiseptic and germicide. Chloroxylenol is used for mildew prevention. Chloroxylenol is also an antibacterial; antiseptic (topical and urinary).
The Uses of 4-Chloro-3,5-dimethylphenol
antibacterial, topical and urinary antiseptic
The Uses of 4-Chloro-3,5-dimethylphenol
Antiseptic and germicide; for mildew prevention. Claimed to be about 60 times as potent as phenol.
Indications
The predominant medical applications for which chloroxylenol is formally indicated for therapeutic use is as an application to the skin for use in cuts, bites, stings, abrasions, and for use as antiseptic hand cleaner .
Background
Chloroxylenol, or para-chloro-meta-xylenol (PCMX), is an antiseptic and disinfectant agent used for skin disinfection and surgical instruments. It is found in antibacterial soaps, wound-cleansing applications, and household antiseptics. The halophenol is shown to be most effective against Gram positive bacteria where it disrupts the cell wall due to its phenolic nature . Chloroxylenol is on the World Health Organization's List of Essential Medicines.
Production Methods
Chloroxylenol is prepared by treating 3,5-dimethylphenol with chlorine or sulfuryl chloride (SO2Cl2).
Definition
ChEBI: 4-chloro-3,5-dimethylphenol is a member of the class of phenols that is 3,5-xylenol which is substituted at position 4 by chlorine. It is bactericidal against most Gram-positive bacteria but less effective against Staphylococci and Gram-negative bacteria, and often inactive against Pseudomonas species. It is ineffective against bacterial spores. It has a role as an antiseptic drug, a disinfectant and a molluscicide. It is a member of phenols and a member of monochlorobenzenes. It is functionally related to a 3,5-xylenol.
Manufacturing Process
546 g of intermediate xylenol fraction having a crystallizing point of 45°C mixed with an equal weight of m-5-xylenol are placed in a suitable vessel, equipped with stirring gear, and 273 g of sulfuryl chloride are added slowly. The temperature rises in the course of the reaction to about 40°C. When all the sulfuryl chloride is added the reaction mixture is heated to 80°C and theacid gases removed as far as possible by air-blowing or any other suitable means. On cooling a quantity of the required chlor-xylenol separates out and is removed from the mother liquor. Further quantities of the material required can be isolated by vacuum distillation of the mother liquors and further crystallization. In all, 200 to 208 g of material substantially 2-chlor-m-5- xylenol can be obtained having a melting point of 112°C to 115°C. The material can be purified if desired by crystallization from a solvent such as a hydrocarbon.
Therapeutic Function
Topical antiseptic; Disinfectant
General Description
The degradation of 4-chloro-3,5-dimethylphenol (PCMX) by Fenton-like process using nanoparticulate zero-valent iron (nZVI) and hydrogen peroxide (H2O2) was studied.
Pharmaceutical Applications
4-Chloro-3,5-dimethylphenol is a common constituent of many proprietary disinfectants used for skin and wound disinfection.
As a pharmaceutical excipient, 4-Chloro-3,5-dimethylphenol is commonly used in low concentrations as an antimicrobial preservative in topical formulations such as creams and ointments. 4-Chloro-3,5-dimethylphenol is also used in a number of cosmetic formulations.
Therapeutically, 4-Chloro-3,5-dimethylphenol has been investigated as a treatment for acne vulgaris, for treating infected root canals, and as an antifungal agent when impregnated into medical devices. 4-Chloro-3,5-dimethylphenol is included in drug products approved for topical antifungal, topical acne and topical dandruff/seborrheic dermatitis/psoriasis treatments.
Agricultural Uses
Microbiocide: 4-Chloro-3,5-dimethylphenol was first registered in the U.S. for use as a fungicide and is now applied as an antimicrobial for control of bacteria, algae and fungi in adhesives, emulsions, paints and wash tanks. It is registered for use in household and domestic dwellings, laundry equipment, bathrooms, diaper pails and in industrial water and aqueous systems. Not listed for use in EU countries. Registered for use in the U.S. There are 64 global suppliers.
Trade name
BAR-FUNGAL PLUS®[C]; BIO SLIME®[C]; DESSON®; DETTOL®; ECOTRU®; ESPADOL®; HUSEPT®; HUSEPT EXTRA®; NIPACIDE®; OTTASEPT®[C]; OTTASEPT® EXTRA[C]; PCMX; RBA 777®
Pharmacokinetics
Chloroxylenol is a substituted phenol which has been widely used for many years as an ingredient of antiseptic and disinfectant products intended for external use . It is known to be bactericidal in low concentration to a wide range of Gram positive and Gram negative bacteria .
Clinical Use
p-Chloro-m-xylenol (PC-MX; Metasep) is a nonirritating antiseptic agent with broad-spectrum antibacterial and antifungal properties. It is marketed in a 2% concentration as a shampoo. It has also been used topically for the treatment of tinea (ringworm) infections such as athlete s foot (tinea pedis) and jock itch (tinea cruris).
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. An eye irritant. An antimicrobial agent. See also CHLOROPHENOLS and CHLORINATED HYDROCARBONS, AROMATIC. When heated to decomposition it emits toxic fumes of Cl-.
Safety
Chloroxylenol is generally regarded as a relatively nontoxic and
nonirritant material when used as an excipient in topical products.
However, chloroxylenol has been placed in Toxicity Category I for
eye irritation effects. In addition, allergic skin reactions have
been reported. Taken orally, chloroxylenol is mildly toxic and
ingestion of a chloroxylenol-containing disinfectant product has
been associated with reports of fatal or severe instances of selfpoisoning.
LD50 (mouse, IP): 0.115 g/kg
LD50 (rat, dermal): >2.0 g/kg
LD50 (rat, oral): 3.83 g/kg
Absorption
No chloroxylenol was detected in the blood following the dermal administration of 2 g of p-chloroxylenol in an ethanol/olive oil vehicle in human subjects . After a dose of 5 g, only traces were found, after 8 g, 1 mg % (1 mg/dL) was found in the blood after 3 hours, and 4 mg % (4 mg/dL) after 24 hours . After a dose of 20 g, 4 mg % (4 mg/dL) was measured after half an hour, and 1 mg % (1 mg/dL) was present at 72 hours .
For antiseptic purposes, chloroxylenol is considered to be well-absorbed when applied to the skin .
Metabolism
Certain animal studies have shown that following dermal application of chloroxylenol, that the absorption was rapid with a Cmax = 1-2 hours, and that the administered substance was excreted via the kidney with almost complete elimination within 24 hours . The primary metabolites discovered in the excreted urine were glucuronides and sulfates . Some chloroxylenol monographs liken its pharmacokinetic profile to that of another antiseptic - triclosan - which is rapidly excreted in the urine also as a glucuronide metabolite, as observed in the human model .
Moreover, In one human subject administered 5 mg intragluteally, 14% was excreted with glucuronic acid and 17% with sulfuric acid at 3 days .
Any chloroxylenol absorbed into the body is likely extensively metabolized by the liver and rapidly excreted, mainly in the urine, as sulphate and glucuronide conjugates .
Synthesis
A method for synthesizing 4-chloro-3, 5-dimethylphenol is characterized by comprising the following steps: under the condition that a cupric salt is used as a catalyst, 3, 5-dimethylphenol, a chlorinating agent and an oxidizing agent are reacted in an organic solvent to obtain 4-chloro-3, 5-dimethylphenol.
storage
Chloroxylenol is stable at normal room temperature, but is volatile in steam. Contact with natural rubber should be avoided. Aqueous solutions of chloroxylenol are susceptible to microbial contamination and appropriate measures should be taken to prevent contamination during storage or dilution. Chloroxylenol should be stored in polyethylene, mild steel or stainless steel containers, which should be well-closed and kept in a cool, dry place. Chloroxylenol does not absorb at wavelengths >290 nm and has been reported to be stable to sunlight for up to 24 hours.
Purification Methods
Crystallise the phenol from *benzene or toluene. [Beilstein 6 IV 3152.]
Incompatibilities
4-Chloro-3,5-dimethylphenol has been reported to be incompatible with nonionic surfactants and methylcellulose.
Regulatory Status
Included in the FDA Inactive Ingredients Database (otic preparations; topical creams and emulsions). Included in nonparenteral medicines licensed in the UK. Approved in Europe as a preservative in cosmetics with a maximum authorized concentration of 0.5%.
Properties of 4-Chloro-3,5-dimethylphenol
| Melting point: | 114-116 °C(lit.) |
| Boiling point: | 246 °C |
| Density | 0.67 g/cm3 |
| refractive index | 1.5523 (estimate) |
| Flash point: | 132 °C |
| storage temp. | 2-8°C |
| solubility | methanol: soluble1g/10 mL, clear to faintly turbid, colorless to very faintly yellow |
| form | Crystalline Powder or Crystals |
| pka | 9.76±0.23(Predicted) |
| color | White to light beige |
| PH | 7 (0.2g/l, H2O) |
| Odor | wh. cryst. or cryst. powd., char. phenolic odor |
| Water Solubility | 0.3 g/L (20 ºC) |
| Merck | 14,2176 |
| BRN | 1862539 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 88-04-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Chloroxylenol(88-04-0) |
| EPA Substance Registry System | 4-Chloro-3,5-dimethylphenol (88-04-0) |
Safety information for 4-Chloro-3,5-dimethylphenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Chloro-3,5-dimethylphenol
| InChIKey | OSDLLIBGSJNGJE-UHFFFAOYSA-N |
4-Chloro-3,5-dimethylphenol manufacturer
Mesha Pharma Limited
KIP Chemicals Pvt Ltd
Super Hygienic Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 88-04-0 98%View Details
88-04-0 98%View Details
88-04-0 -
 Chloroxylenol 88-04-0 98%View Details
Chloroxylenol 88-04-0 98%View Details
88-04-0 -
 p-Chloro m-xylenol CAS 88-04-0View Details
p-Chloro m-xylenol CAS 88-04-0View Details
88-04-0 -
 4-Chloro-3,5-dimethylphenol CAS 88-04-0View Details
4-Chloro-3,5-dimethylphenol CAS 88-04-0View Details
88-04-0 -
 4-CHLORO-m-XYLENOL Extra Pure CAS 88-04-0View Details
4-CHLORO-m-XYLENOL Extra Pure CAS 88-04-0View Details
88-04-0 -
 88-04-0 98%View Details
88-04-0 98%View Details
88-04-0 -
 Chloroxylenol 98% CAS 88-04-0View Details
Chloroxylenol 98% CAS 88-04-0View Details
88-04-0 -
 Chloroxylenol CAS 88-04-0View Details
Chloroxylenol CAS 88-04-0View Details
88-04-0