4-Chloro-2-methylphenol
Synonym(s):4-Chloro-o-cresol
- CAS NO.:1570-64-5
- Empirical Formula: C7H7ClO
- Molecular Weight: 142.58
- MDL number: MFCD00002321
- EINECS: 216-381-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
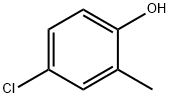
What is 4-Chloro-2-methylphenol?
Chemical properties
Off-white to slightly brownish crystalline solid
The Uses of 4-Chloro-2-methylphenol
4-Chloro-2-methylphenol is a transformation/degredation product of herbicides found in the environment through landfills and runoff.
Definition
ChEBI: 4-chloro-2-methylphenol is a member of the class of phenols that is o-cresol in which the hydrogen para to the hydroxy group is replaced by a chlorine. It is a member of phenols and a member of monochlorobenzenes. It is functionally related to an o-cresol.
What are the applications of Application
4-Chloro-2-methylphenol is an important pharmaceutical intermediate ingredient used in the manufacture of herbicides (Mecoprop, MCPA, and MCPB), as an additive tracer for resist plasma etching. It is used as a closed system intermediate in the production of phenoxy herbicides. It is also used as a disinfectant or bactericide.
Synthesis Reference(s)
Tetrahedron Letters, 30, p. 5215, 1989 DOI: 10.1016/S0040-4039(01)93745-1
General Description
Dark red flakes with waxy texture. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
4-Chloro-2-methylphenol can react vigorously with concentrated sodium hydroxide solutions. Also reacts with other bases, acid chlorides, acid anhydrides, and oxidizing agents. Corrodes steel, brass, copper and copper alloys [NTP, 1992)]. A large quantity left in contact with concentrated sodium hydroxide solution for 3 days reacted violently, attaining red heat and evolving fumes that ignited explosively. The heat of reaction dissipated poorly because of the high viscosity of the mixture [Quart. Safety Summ., 1957, 28, 39].
Fire Hazard
4-Chloro-2-methylphenol is probably combustible.
Flammability and Explosibility
Non flammable
Purification Methods
Purify the phenol by crystallisation from pet ether (m 51o) and by zone melting. [Beilstein 6 H 359, 6 I 174, 6 II 332, 6 III 1264, 6 IV 1987.]
Properties of 4-Chloro-2-methylphenol
| Melting point: | 43-46 °C(lit.) |
| alpha | 22 º (c=8,6N HCl) |
| Boiling point: | 220-225 °C(lit.) |
| Density | 1.20 |
| vapor pressure | 26.66Pa at 19.85℃ |
| refractive index | 1.5449 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| form | neat |
| pka | 9.87±0.18(Predicted) |
| color | white to brown |
| Water Solubility | <0.1 g/100 mL at 15 ºC |
| BRN | 1906684 |
| CAS DataBase Reference | 1570-64-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-chloro-2-methyl-(1570-64-5) |
| EPA Substance Registry System | 4-Chloro-2-methylphenol (1570-64-5) |
Safety information for 4-Chloro-2-methylphenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Chloro-2-methylphenol
| InChIKey | RHPUJHQBPORFGV-UHFFFAOYSA-N |
4-Chloro-2-methylphenol manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1570-64-5 4 – CHLORO – O – CRESOL 98%View Details
1570-64-5 4 – CHLORO – O – CRESOL 98%View Details
1570-64-5 -
 1570-64-5 98%View Details
1570-64-5 98%View Details
1570-64-5 -
 4-Chloro-2-methylphenol CAS 1570-64-5View Details
4-Chloro-2-methylphenol CAS 1570-64-5View Details
1570-64-5 -
 4-Chloro-o-cresol CAS 1570-64-5View Details
4-Chloro-o-cresol CAS 1570-64-5View Details
1570-64-5 -
 4-Chloro-2-methylphenol CAS 1570-64-5View Details
4-Chloro-2-methylphenol CAS 1570-64-5View Details
1570-64-5 -
 4-Chloro-2-methylphenol CAS 1570-64-5View Details
4-Chloro-2-methylphenol CAS 1570-64-5View Details
1570-64-5 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1