m-Cresol
Synonym(s):m-Cresol;3-Methylphenol;m-Cresol;m-Cresol solution;1-Hydroxy-3-methylbenzene
- CAS NO.:108-39-4
- Empirical Formula: C7H8O
- Molecular Weight: 108.14
- MDL number: MFCD00002302
- EINECS: 203-577-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
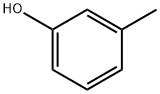
What is m-Cresol ?
Chemical properties
colourless to light yellow liquid
Chemical properties
Cresol is a mixture of the three isomeric cresols, o-, m-, and p-cresol. Cresols are slightly soluble in water. m-Isomer: Colorless or yellow liquid with characteristic odor.
Chemical properties
m-Cresol has a dry, tarry, medicinal–leathery odor. m-Cresol and p-cresol very often occur together and are difficult to separate.
Occurrence
Reported found in beer, coffee, egg, grape, Oriental tobacco, roasted barley, rum, sherry and whiskey.
The Uses of m-Cresol
m-Cresol is used as a disinfectant and solvent. LysolTM disinfectant is a 50% (v/v) mixed-cresol isomer in a soap emulsion formed on mixing with water. The isomer m-cresol is an oily liquid with low volatility. Besides disinfection at solutions of 1–5%, the cresols are used in degreasing compounds, paintbrush cleaners, and additives in lubricating oils. Cresols were once widely used for disinfection of poultry houses, but this use has been discontinued because they cause respiratory problems and abdominal edema in young chicks. m-Cresol has been used in synthetic resins, explosives, petroleum, photographic, paint, and agricultural industries.
The Uses of m-Cresol
Antiseptic; antimicrobial preservative.
The Uses of m-Cresol
m-Cresol is employed in the preparation of synthetic resins like phenol-formaldehyde resins, disinfectants, and photographic developers. It is used as industrial solvent for dissolving polymers such as polyaniline. It acts as a precursor to vitamin E and antiseptic amylmetacresol. It is used as insuline preservatives.
Definition
ChEBI: A cresol with the methyl substituent at position 3. It is a minor urinary metabolite of toluene.
Preparation
One process involving butylation and separation and followed by dealkylation produces m-cresol and ditertiary butyl p-cresol. From m, p-cresol (which is the main product of the recovery process) pure m-cresol can be obtained by extraction.
Production Methods
The cresols (cresylic acids) are methyl phenols and generally appear as a mixture of isomers. m-Cresol is prepared from m-toluic acid or obtained from coal tar or petroleum (352, 355, 356). Crude cresol is obtained by distilling “gray phenic acid” at a temperature of ~180–201°C. The m-cresol may be separated from the crude or purified mixture by repeated fractional distillation in vacuo. It can also be prepared synthetically by diazotization of the specific toluidine or by fusion of the corresponding toluenesulfonic acid with sodium hydroxide.
Aroma threshold values
Aroma characteristics at 1.0%: phenolic, spicy eugenol-like, medicinal, smoky powdery with a leatherlike note.
Taste threshold values
Taste characteristics at 2 ppm: phenolic, smoky, balsamic, medicinal and spicy eugenol-like
General Description
Colorless liquid with a sweet tarry odor. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
m-Cresol is sensitive to air, light and heat. m-Cresol is also hygroscopic. m-Cresol can react vigorously with strong oxidizers and strong bases. m-Cresol reacts violently with nitric acid, oleum, chlorosulfonic acid, metals and strong acids. If the water content is below approximately 0.3% and the temperature above 248° F, the corrosion of aluminum and its alloys may occur violently. m-Cresol will attack some forms of plastics, coatings and rubber.
Hazard
Questionable carcinogen.
Health Hazard
INHALATION: Mucosal irritation and systemic poisoning EYES: Intense irritation and pain, swelling of conjunctiva, and corneal damage may occur. SKIN: Intense burning, loss of feeling, wrinkling, white discoloration, and softening. Gangrene may occur. INGESTION: Burning sensation in mouth and esophagus. Vomiting may result. Acute exposure by all routes may cause muscular weakness, gastroenteric disturbances, severe depression, collapse. Effects are primarily on CNS and edema of lungs. Injury of spleen and pancreas may occur.
Flammability and Explosibility
Not classified
Contact allergens
Metacresol is contained as a preservative in almost all human insulin. It has been reported as a cause of allergic reaction due to injected insulin.
Safety Profile
v Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by skin contact. Severe eye and skin irritant. An experimental teratogen. Human mutation data reported. Questionable carcinogen with experimental neoplastigenic data. Flammable when exposed to heat or flame. Moderately explosive in the form of vapor when exposed to heat or flame. See also other cresol entries and PHENOL.
Potential Exposure
Cresol is used as a disinfectant and fumigant; as an ore flotation agent, and as an intermediate in the manufacture of chemicals, dyes, plastics, and antioxidants. A mixture of isomers is generally used; the concentrations of the components are determined by the source of the cresol.
Carcinogenicity
m-Cresol has induced a few papillomas but no carcinomas in tumor studies.
Shipping
UN2076 Cresols, liquid, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN3455 Cresols, solid, Hazard class: 6.1; Labels: 6.1- Poisonous materials, 8-Corrosive material.
Purification Methods
Separation of the m-and p-cresols requires chemical methods, such as conversion to their sulfonates [Brüchner Anal Chem 75 289 1928]. An equal volume of H2SO4 is added to m-cresol, stirred with a glass rod until solution is complete. Heat for 3hours at 103-105o. Dilute carefully with 1-1.5 volumes of water, heat to boiling point and steam distil until all unsulfonated cresol has been removed. Cool and extract the residue with ether. Evaporate the solution until the boiling point reaches 134o and steam distil off the m-cresol. Another purification method involves distillation, fractional crystallisation from the melt, then redistillation. Free from p-cresol by solution in glacial acetic acid and bromination by about half of an equivalent amount of bromine in glacial acetic acid. The acetic acid is distilled off, then fractional distillation of the residue under vacuum gives bromocresols from which 4-bromo-m-cresol is obtained by crystallisation from hexane. Addition of the bromocresol in glacial acetic acid slowly to a reaction mixture of HI and red phosphorus or (more smoothly) of HI and hypophosphorus acid, in glacial acetic acid, at reflux, removes the bromine. After an hour, the solution is distilled at atmospheric pressure until layers are formed. Then it is cooled and diluted with water. The cresol is extracted with ether, washed with water, NaHCO3 solution and again with water, dried with a little CaCl2 and distilled [Baltzly et al. J Am Chem Soc 77 2522 1955]. The 3,5-dinitrobenzoate (prepared with 3,5-dinitrobenzoyl chloride in dry pyridine, and recrystallised from EtOH or aqueous Me2CO) has m 165o. [Beilstein 6 IV 2035.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with strong acids; oxidizers, alkalies, aliphatic amines; amides, chlorosulfonic acid; oleum. Decomposes on heating, producing strong acids and bases, causing fire and explosion hazard. Liquid attacks some plastics and rubber. Attacks many metals.
Waste Disposal
Wastewaters may be subjected to biological treatment. Concentrations may be further reduced by ozone treatment. High concentration wastes may be destroyed in special waste incinerators.
Properties of m-Cresol
| Melting point: | 8-10 °C(lit.) |
| Boiling point: | 203 °C(lit.) |
| Density | 1.034 g/mL at 25 °C(lit.) |
| vapor density | 3.72 (vs air) |
| vapor pressure | <1 mm Hg ( 20 °C) |
| FEMA | 3530 | M-CRESOL |
| refractive index | n |
| Flash point: | 187 °F |
| storage temp. | Store below +30°C. |
| solubility | 23.5g/l |
| form | Liquid |
| pka | 10.01(at 25℃) |
| color | Clear colorless to slight red |
| PH | 5 (20g/l, H2O, 20℃) |
| Odor | Dry-tarry, medicinal-leathery odor |
| Odor Threshold | 0.0001ppm |
| explosive limit | 1.06-1.35%, 150°F |
| Water Solubility | 20 g/L (20 ºC) |
| λmax | 273nm(lit.) |
| JECFA Number | 692 |
| Merck | 14,2579 |
| BRN | 506719 |
| Exposure limits | ACGIH: TWA 20 mg/m3 (Skin) NIOSH: IDLH 250 ppm; TWA 2.3 ppm(10 mg/m3) |
| Dielectric constant | 11.5 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases, strong acids, aluminium, aluminium alloys. Air and light sensitive. Hygroscopic. |
| CAS DataBase Reference | 108-39-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 3-methyl-(108-39-4) |
| EPA Substance Registry System | m-Cresol (108-39-4) |
Safety information for m-Cresol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for m-Cresol
m-Cresol manufacturer
Antares Chem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 m-Cresol CAS 108-39-4View Details
m-Cresol CAS 108-39-4View Details
108-39-4 -
 m-Cresol extrapure AR CAS 108-39-4View Details
m-Cresol extrapure AR CAS 108-39-4View Details
108-39-4 -
 m-Cresol pure CAS 108-39-4View Details
m-Cresol pure CAS 108-39-4View Details
108-39-4 -
 Meta Cresol CASView Details
Meta Cresol CASView Details -
 Meta CresolView Details
Meta CresolView Details
108-39-4 -
 M-Cresol Chemical, For IndustrialView Details
M-Cresol Chemical, For IndustrialView Details
108-39-4 -
 Technical Grade M-Cresol Liquid, Packaging Size: 180 kgsView Details
Technical Grade M-Cresol Liquid, Packaging Size: 180 kgsView Details
108-39-4 -
 META CRESOL (CAS NO.108-39-4)View Details
META CRESOL (CAS NO.108-39-4)View Details
106-44-5
