4-Bromo-1H-indazole
Synonym(s):4-Bromoindazole
- CAS NO.:186407-74-9
- Empirical Formula: C7H5BrN2
- Molecular Weight: 197.03
- MDL number: MFCD05664001
- EINECS: 803-241-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
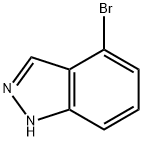
What is 4-Bromo-1H-indazole?
Description
4-Bromo-1H-indazole is a derivative indazole which has a heterocyclic structure made up of benzene and pyrazole rings. Indazoles are an important class of heterocyclic compounds with a wide range of application as biologic and pharmaceutic agents. Indazole derivatives have a wide range of biological activities. For instance, indazole derivatives show vasorelaxant and anti-aggregator activities by stimulating NO release and increasing cGMP levels. Recent medical research and development studies resulted in the production of indazole derivatives for the treatment of osteoporosis, inflammatory diseases and neurodegenerative disorders. 4-bromo-1H indazole molecule is a much stronger inhibitor of 6-bromo-1H indazole and 7-bromo-1H indazole. 4-Bromo-1H-indazole may be strong inhibitor candidate for LPO and that caution should be exercised when using medicines containing indazole derivatives[1].
Chemical properties
brown powder
Synthesis
To N-Acetyl-4-bromo-1H-indazole (1.6 g, 6.69 mmol) was added methanol (25.9 mL) and then 6 N HCl (15.6 mL). The resulting solution was stirred at room temperature until the reaction was completed after 7 h (monitored by TLC, petroleum ether/ethyl acetate 2:1). After evaporation of methanol in vacuo, the residue was extracted with ethyl acetate (50 mL*3). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude was purified by flash chromatography (petroleum ether/ethyl acetate, 5:1) to yield 4-bromo-1H-indazole (0.8 g) as milky white solid, mp 164–166°C, yield 67.1%[2].
References
[1] Koksal Z, et al. Lactoperoxidase, an antimicrobial enzyme, is inhibited by some indazoles. Drug and Chemical Toxicology, 2018; 43: 22-26.
[2] Wang Y, et al. Synthesis and Antibacterial Activity of Novel 4-Bromo-1H-Indazole Derivatives as FtsZ Inhibitors. Archiv der Pharmazie, 2015; 348: 266-274.
Properties of 4-Bromo-1H-indazole
| Melting point: | 165-167° |
| Boiling point: | 333.8±15.0 °C(Predicted) |
| Density | 1.770±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | soluble in Ethanol |
| form | Solid |
| pka | 12.78±0.40(Predicted) |
| color | Off-white |
| InChI | InChI=1S/C7H5BrN2/c8-6-2-1-3-7-5(6)4-9-10-7/h1-4H,(H,9,10) |
| CAS DataBase Reference | 186407-74-9(CAS DataBase Reference) |
Safety information for 4-Bromo-1H-indazole
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Bromo-1H-indazole
| InChIKey | KJIODOACRIRBPB-UHFFFAOYSA-N |
| SMILES | N1C2=C(C(Br)=CC=C2)C=N1 |
4-Bromo-1H-indazole manufacturer
SYNARTIS PHARMA PVT LTD
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran







You may like
-
 186407-74-9 4-bromo-1H-indazole NLT-98%View Details
186407-74-9 4-bromo-1H-indazole NLT-98%View Details
186407-74-9 -
 4-Bromoindazole CAS 186407-74-9View Details
4-Bromoindazole CAS 186407-74-9View Details
186407-74-9 -
 4-Bromo-1H-indazole CAS 186407-74-9View Details
4-Bromo-1H-indazole CAS 186407-74-9View Details
186407-74-9 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
