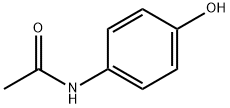
4-Aminopyridine
Synonym(s):4-Aminopyridine;4AP;4-Pyridinamine;4-Pyridylamine;Fampridine
- CAS NO.:504-24-5
- Empirical Formula: C5H6N2
- Molecular Weight: 94.11
- MDL number: MFCD00006439
- EINECS: 207-987-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-14 12:08:39
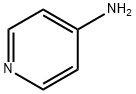
What is 4-Aminopyridine?
Absorption
Orally-administered dalfampridine is rapidly and completely absorbed from the gastrointestinal tract. Tmax, immediate release form = 1 hour; Tmax, extended release form = 3.5 hours; Cmax, 10 mg extended release = 17.3 - 21.6 ng/mL; Relative bioavailability of 10 mg extended-release tablets compared to aqueous oral solution = 96%
Toxicity
LD50, oral, mouse = 19 mg/kg LD50, oral, rat = 21 mg/kg
Description
4-Aminopyridine (4-AP, fampridine, dalfampridine) is the first drug approved by the FDA to improve walking in patients with multiple sclerosis (MS). Dalfampridine is a voltage-gated potassium channel blocker that readily penetrates the CNS and increases the conduction and duration of action potential across nerve fibers resulting in enhanced functionality as observed in the walking speed of MS patients.
From a safety perspective, seizure was the most important adverse event from various dalfampridine clinical trials. Therefore, dalfampridine is contraindicated in patients with a history of seizures. Since dalfampridine is primarily excreted by the kidney as unchanged drug, it is also contraindicated in patients with moderate to severe renal impairment. Assessing the overall benefit–risk profile from various clinical trials, the FDA approved dalfampridine (daily oral dose of 10 mg, b.i.d.) to improve walking in MS patients with existing gait impairment.
Chemical properties
4-aminopyridine is a white crystalline solid that contains about 98% active ingredient. It is soluble in water and slightly soluble in benzene and ether. 4-Aminopyridine formulations are classifi ed by the US Environmental Protection Agency (US EPA) as restricted use pesticides (RUPs) and therefore should be purchased and used only by certifi ed and trained workers and applicators. Avitrol is available as grain baits or as a powder concentrate. It is one of the most prominent avicides. It is registered with the EPA for use against red-winged blackbirds, blackbirds in agricultural fi elds, grackles, pigeons, and sparrows around public buildings, and various birds around livestock feeding pens. Avitrol repels birds by poisoning a few members of a fl ock, causing them to become hyperactive.
Originator
Rush University Medical Center (United States)
The Uses of 4-Aminopyridine
Avitrol (4-aminopyridine), has repellent–toxicant properties for birds and is classed as a severe poison and irritant. This secondary bird repellent can be used as a broadcast bait, causing uncoordinated flight and distress calls and escape responses in nearby birds.
The Uses of 4-Aminopyridine
Dalfampridine is used in characterizing subtypes of potassium channel, and has also been used to manage some of the symptoms of multiple sclerosis, and is indicated for symptomatic improvement of walking in adults with several variations of the disease. Dalfampridine is a precursor to the drug Pinacidil.
Background
Dalfampridine is a potassium channel blocker used to help multiple sclerosis patients walk. This is the first drug that was specifically approved to help with mobility in these patients. FDA approved on January 22, 2010.
Indications
Dalfampridine is a neurofunctional modifier that helps improve walking speed in patients with multiple sclerosis (MS).
What are the applications of Application
4-Aminopyridine is a nonselective K+ channel blocker that binds from the cytoplasmic side of the cell membrane
Definition
ChEBI: 4-aminopyridine is an aromatic amine that is pyridine bearing a single amino substituent at position 4. An orphan drug in the US, it is used to improve walking in adults with multiple sclerosis. It has a role as an avicide, a potassium channel blocker and an orphan drug. It is an aromatic amine and an aminopyridine.
brand name
Neurelan (E′lan);Ampyra.
Synthesis Reference(s)
Tetrahedron Letters, 39, p. 1313, 1998 DOI: 10.1016/S0040-4039(97)10877-2
General Description
White crystalline material with no odor. Used as an avicide, an intermediate and as a fixer for some textile dyes.
Reactivity Profile
4-Aminopyridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
4-Aminopyridine, a pyridine compound, is an extremely effective bird poison. In agriculture, 4-AP is used as an extremely effective bird poison sold under the brand name Avitrol. It is highly toxic to all mammals including humans if dosages are exceeded, and as an experimental drug, the recommended dose data is unavailable.
Fire Hazard
Material may produce irritating or poisonous gases in fire. Runoff from fire control water may give off irritating or poisonous gases.
Pharmacokinetics
Dalfampridine is a board-spectrum lipophillic potassium channel blocker and binds favourably to the open state than closed state of the potassium channel in the CNS. Its pharmacological target are the potassium channels exposed in MS patients. Does not prolong the QTc interval.
Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: hallucinations and vomiting. When heated to decomposition it emits toxic fumes of NOx,.
Potential Exposure
Used as a chemical intermediate in pharmaceuticals; as an agricultural chemical for field crops; and as a bird repellent and poison
in vitro
4-ap acts by selectively blocking fast, voltage-gated k+ channels in excitable tissues. in axons, k+ channel blockade increases the safety factor1 across demyelinated internodes and 4-ap can, therefore, restore conduction in focally demyelinated axons. 4-ap also increases calcium (ca2+) influx at presynaptic terminals thereby enabling an enhancement of neuroneuronal or neuromuscular transmission in normally myelinated neurons [1].
in vivo
investigations of the effects of 4-ap on neurologic deficits in animal in vivo models of demyelinating disease or sci have yielded inconsistent results. some trials have shown indications of potential neurological benefit, such as enhanced motor evoked potentials or reflex activity, while others have yielded no evident gains in function [1].
Metabolism
Not extensively metabolized by the liver therefore drugs effecting the cytochrome P450 enzyme system that are concomitantly administered with dalfampridine are not expected to interact with each other. Metabolites include 3-hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate and both are inactive. CYP2E1 is the enzyme responsible for 3-hydroxylation of dalfampridine.
Storage
Room temperature
Shipping
UN2671 Aminopyridines, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise the aminopyridine from *benzene/EtOH, then recrystallise it twice from water, then crush and dry it for 4hours at 105o [Bates & Hetzer J Res Nat Bur Stand 64A 427 1960]. It has also been crystallised from EtOH, *benzene, *benzene/pet ether, toluene and sublimes in a vacuum. [Beilstein 22/9 V 106.]
Toxicity evaluation
4-Aminopyridine is highly toxic to animals, with an approximate oral LD50 of 3.7 mg/kg body weight in the dog. 4-Aminopyridine blocks potassium channels, resulting in increased cholinergic nervous system activity. The ability of 4-aminopyridine to block potassium channels has led to some speculation that it may be an effective therapy for multiple sclerosis. Clinical signs in poisoned animals often develop within several hours of ingestion and commonly include tachycardia, salivation, tremors, ataxia, and seizures. Death from respiratory failure may develop within 4 hours of exposure. Chemical analysis of frozen stomach contents, liver, and urine is available at some diagnostic laboratories, and residues can be detected in poisoned birds. Although no specifi c antidotal therapy exists, anticonvulsants and activated charcoal administration are recommended.
Incompatibilities
Sodium nitrite, strong oxidizers. Avoid contact with acid anhydrides, acid chlorides; and strong acids.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposalIncineration with nitrogen oxides removal from effluent gas.
References
[1] hayes kc. the use of 4-aminopyridine (fampridine) in demyelinating disorders. cns drug rev. 2004 winter;10(4):295-316.
Properties of 4-Aminopyridine
| Melting point: | 155-158 °C (lit.) |
| Boiling point: | 273 °C (lit.) |
| Density | 1.26 |
| vapor pressure | 0.046Pa at 25℃ |
| refractive index | 1.5560 (estimate) |
| Flash point: | 156°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble50mg/mL, clear, colorless |
| form | Crystalline Powder, Solid or Crystals |
| pka | 9.114(at 25℃) |
| color | White to faint grey to light yellow |
| Water Solubility | Soluble in water(112 g/L at 20 deg C). Soluble in ethyl ether, benzene. Slightly soluble in ligroin. Very soluble in ethanol. Soluble in methanol, acetone, tetrahydrofuran, isopropanol, acetonitrile, N,N-dimethylformamide, dimethylsulfoxide, and ethanol. |
| Merck | 14,3933 |
| BRN | 105782 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 504-24-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Pyridinamine(504-24-5) |
| EPA Substance Registry System | 4-Aminopyridine (504-24-5) |
Safety information for 4-Aminopyridine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Aminopyridine
| InChIKey | NUKYPUAOHBNCPY-UHFFFAOYSA-N |
4-Aminopyridine manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 504-24-5 4-Aminopyridine 98%View Details
504-24-5 4-Aminopyridine 98%View Details
504-24-5 -
 4-Amino pyridine CAS 504-24-5View Details
4-Amino pyridine CAS 504-24-5View Details
504-24-5 -
 4-Aminopyridine CAS 504-24-5View Details
4-Aminopyridine CAS 504-24-5View Details
504-24-5 -
 Dalfampridine CAS 504-24-5View Details
Dalfampridine CAS 504-24-5View Details
504-24-5 -
 4-AMINOPYRIDINE For Synthesis CAS 504-24-5View Details
4-AMINOPYRIDINE For Synthesis CAS 504-24-5View Details
504-24-5 -
 4 Amino Pyridine Cas 504 24 5View Details
4 Amino Pyridine Cas 504 24 5View Details
504-24-5 -
 4 Amino Pyridine Cas 504 24 5View Details
4 Amino Pyridine Cas 504 24 5View Details
504-24-5 -
 4 Amino Pyridine Chemical, Grade Standard: Lab GradeView Details
4 Amino Pyridine Chemical, Grade Standard: Lab GradeView Details
110-86-1
