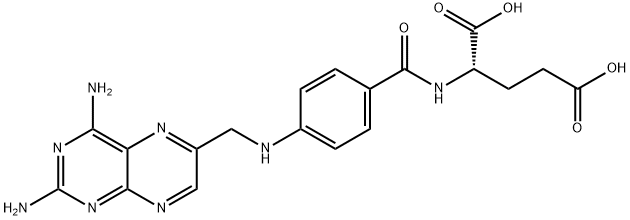
4-AMINOFOLIC ACID HYDRATE
- Empirical Formula: C19H22N8O6
- Molecular Weight: 458.43
- MDL number: MFCD00150465

What is 4-AMINOFOLIC ACID HYDRATE?
Originator
Aminopterin,Sigma Chemical Company
Manufacturing Process
2,4,5,6-Tetraaminopyrimidine·H2SO4·H2O (75.0 g, 0.293 mole) was added to a
stirred solution of BaCl2·2H2O (71.5 g, 0.293 mole) in H2O (1.45 L) at 85-
90°C. The mixture was stirred rapidly at about 90°C for 15 min, cooled to
40°C, and filtered from BaSO4, which was washed thoroughly on a funnel withH2O. The clear, yellow filtrate was then diluted further with H2O to give a
volume of 4.35 L. This solution of the tetraaminopyrimidine·2HCl was then
added to a solution of NaOAc (4.35 L of 4 N) in which 1,3-dihydroxyacetone
(79.3 g, 0.88 mole) and cysteine·HCl·H2O (51.5 g, 0.293 mole) had just been
dissolved. The resulting solution was stirred mechanically at room
temperature while a slow stream of air was continuously passed through it for
26 hours. (Yellow-orange solid began separating after 2 hours). The mixture
was then kept in a refrigerator for 16 hours before the solid was collected,
washed successively with cold H2O, EtOH, and Et2O before it was dried to
constant weight in vacuo over P2O5 at 25°C. [The crude product mixture (47
g) was weighed in order to obtain an estimate of the volume of 48% HBr
required to form hydrobromide salts]. A mechanically stirred mixture of the
dried solid and EtOH (6.05 L) was heated to 70°C, and a solution of 48% HBr
(28 ml) in EtOH (490 ml) was added in a thin stream while the mixture was
maintained at 70-75°C. The mixture was then refluxed for about 5 min with
rapid stirring while nearly all of the solid dissolved. The hot solution was
treated with Norit and filtered through a Celite mat. The clear yellow filtrate
was kept in a refrigerator overnight while a first crop of orange colored solid
separated. The collected solid was washed with EtOH, then dried in vacuo
(56°C over P2O5) to give 17.2 g of product. The filtrate was concentrated by
evaporation (rotary evaporator) to about 2 L and then refrigerated to give a
second crop of 10.2 g, which was dried as before; total yield of crude 2,4-
diamino-6-pteridinemethanol hydrobromide 27.4 g (34%). The PMR spectrum
of this material in CF3CO2D showed it to contain a barely detectable amount
of methyl-substituted 2,4-diaminopteridine·HBr.
Bromine (59.6 g, 0.373 mole) was added dropwise over a 30 min to a stirred
solution of triphenylphosphine (97.7 g, 0.373 mole) in anhydrous
dimethylacetamide (486 ml) kept at 10°C (ice bath) and protected from
atmospheric moisture. (Bromine remaining in the funnel was rinsed with 10
ml of dimethylacetamide). A smooth suspension containing finely divided,
crystalline triphenylphosphine dibromide resulted. The 2,4-diamino-6-
pteridinemethanol·HBr (25.4 g, 0.093 mole) described above was added in
one portion through a powder funnel (with the aid of 10 ml
dimethylacetamide). The ice bath was removed, and the stirred mixture was
allowed to warm to 20-25°C. After about 1 hour, complete solution had
occurred. The solution, which gradually developed a dark red color, was kept
at 20-25°C for 1 hour longer and was then chilled (ice bath) before it was
treated with EtOH (72 ml). After overnight refrigeration, the solvents were
removed by evaporation in vacuo. The dark, semisolid residue was stirred with
two 300 ml of C6H6 (to remove triphenylphosphine oxide), and each portion
was removed from the C6H6 insoluble product by decantation. The solid that
remained was dissolved with stirring in glacial AcOH (660 ml) which had been
preheated to 80°C. The mixture was kept in a bath at 80°C until solution was
complete. Tan crystalline solid separated as the dark solution was allowed to
cool. Overnight refrigeration caused the AcOH to partially freeze. When it had
thawed, the solid was collected, washed with chilled AcOH followed by Et2O,
and dried in vacuo (over P2O5 and NaOH pellets) at successive temperatures
of 25°C, 56°C, and 110°C. (The higher temperature was necessary for
complete removal of AcOH). The yield was 15.3 g (49%). (Some runs afforded
60% yield). This sample was further purified by reprecipitation from MeOH
solution (Norit) by addition of Et2O followed by drying in vacuo (25°C, P2O5),
yield 13.0 g (42%) of 2,4-diamino-6-(bromomethyl)pteridine hydrobromide as A mixture of 2,4-diamino-6-(bromomethyl)pteridine hydrobromide (168 mg,
0.500 mmole) and N-(4-aminobenzoyl)-L-glutamic acid (400 mg, 1.50
mmoles) in dimethylacetamide (2 ml) was stirred at 25°C under N2 in a
stoppered flask protected from light. Solution occurred after 2 hours. After 18
hours, the orange solution was mixed with H2O (15 ml) with stirring to give a
finely divided, yellow precipitate. The mixture was centrifuged, and the
supernatant removed by decantation. The yellow solid was stirred with four 15
ml portions of H2O, each of which was removed by decantation after
centrifugation. The solid was then suspended in EtOH (15-20 ml), collected by
filtration, washed with Et2O, and dried in vacuo (25°C, P2O5) to give hydrated
N-[4-[[(2,4-diamino-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic acid
(Aminopterin) hydrate (4:7) in 68% yield (160 mg). Examination by TLC
revealed one UV-absorbing spot and no fluorescence at any point.
Therapeutic Function
Antineoplastic
Properties of 4-AMINOFOLIC ACID HYDRATE
| Melting point: | 225 °C (dec.)(lit.) |
Safety information for 4-AMINOFOLIC ACID HYDRATE
Computed Descriptors for 4-AMINOFOLIC ACID HYDRATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8