4-Aminobenzaldehyde
- CAS NO.:556-18-3
- Empirical Formula: C7H7NO
- Molecular Weight: 121.14
- MDL number: MFCD00038137
- EINECS: 209-115-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-05 20:24:49
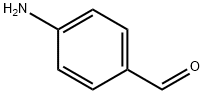
What is 4-Aminobenzaldehyde?
Description
4-Aminobenzaldehyde can be made into polymer with a certain conductivity and corrosion resistance, and the combination of stainless steel is better than the epoxy resin. It can be used as intermediate of pharmaceutical and dye. It is also used in organic synthesis. Additionally, it can react with Carbonyl dichloride to get 4-Formylphenyl isocyanate.
Chemical properties
Yellow crystalline powder. Melting point 71-72℃. Soluble in alcohol, benzene, insoluble in water, very easy to polymerize.
The Uses of 4-Aminobenzaldehyde
4-Aminobenzaldehyde (p-aminobenzaldehyde) is a useful synthetic reagent and monomer that can be used to synthesize monoazo dyes and photocurable ion exchange resins. 4-Aminobenzaldehyde is also a corrosion inhibitor of metals.
What are the applications of Application
4-Aminobenzaldehyde is a useful synthetic reagent and monomer
Definition
ChEBI: P-aminobenzaldehyde is a member of benzaldehydes.
Preparation
4-aminobenzaldehyde is obtained from p-nitrotoluene by oxidation, reduction, sodium sulfide, and sodium hydroxide dissolved in water, filtration, and filtrate heated to 68 ° C to add sulfur powder, at 98 ° C reaction for 18min to get more sodium sulfide solution. Add 65% ethanol and p-nitrotoluene, reaction at 80-86 ℃ for 1.5h, recovery of ethanol, and steam distillation to remove by-products p-aminotoluene. The reaction solution is extracted with benzene, distilled by water vapor, cooled, filtered, and dried to obtain the finished product.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 4, p. 31, 1963
Tetrahedron Letters, 30, p. 251, 1989 DOI: 10.1016/S0040-4039(00)95173-6
References
[1] O. A. NURKENOV. Synthesis, structure and chemical transformations of 4-aminobenzaldehyde[J]. Russian Journal of General Chemistry, 2013, 83 10: 1864-1868. DOI:10.1134/S1070363213100113.
[2] MD. J. SHARIF Tatsuya T Seiji Yamazoe. Selective Hydrogenation of 4-Nitrobenzaldehyde to 4-Aminobenzaldehyde by Colloidal RhCu Bimetallic Nanoparticles[J]. Topics in Catalysis, 2014, 57 10-13: 1049-1053. DOI:10.1007/s11244-014-0269-5.
[3] KUNIO KIMURA. Self-Assembling Polycondensation of 4-Aminobenzaldehyde. Preparation of Star-Like Aggregates of Cone-Shaped Poly(azomethine) Crystals[J]. Polymer Journal, 2003, 35 5: 455-459. DOI:10.1295/polymj.35.455.
[4] A. HALSTIANYe. Yu V A S Вushuiev. Ozonation of 4-aminotoluene as a new method of synthesis of 4-aminobenzaldehyde – an intermediate for the production of anti-tuberculosis drugs[J]. Current issues in pharmacy and medicine: science and practice, 2022, 18 1. DOI:10.14739/2409-2932.2022.1.249620.
[5] Lei Z U .Preparation and Application of 4-Aminobenzaldehyde and Its Polymer[J].Journal of Yanbian University(Natural Science), 2008.
Properties of 4-Aminobenzaldehyde
| Melting point: | 77-79°C |
| Boiling point: | 138-139 C |
| Density | 0,868 g/cm |
| refractive index | 1.5323 (estimate) |
| pka | 1.88±0.10(Predicted) |
| form | Solid |
| color | Light yellow to yellow |
| CAS DataBase Reference | 556-18-3(CAS DataBase Reference) |
| EPA Substance Registry System | Benzaldehyde, 4-amino- (556-18-3) |
Safety information for 4-Aminobenzaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Aminobenzaldehyde
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 4-Aminobenzaldehyde 98%View Details
4-Aminobenzaldehyde 98%View Details
556-18-3 -
 4-Aminobenzaldehyde, 95% CAS 556-18-3View Details
4-Aminobenzaldehyde, 95% CAS 556-18-3View Details
556-18-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
