4-(2-Butyl)phenol
- CAS NO.:99-71-8
- Empirical Formula: C10H14O
- Molecular Weight: 150.22
- MDL number: MFCD00002375
- EINECS: 202-781-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
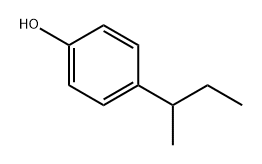
What is 4-(2-Butyl)phenol?
Chemical properties
The butylphenols include several isomers. Solid butylphenols (28805-86-9) generally have properties similar to the above:
The Uses of 4-(2-Butyl)phenol
Phenolic Resins, Epoxy Resins
Definition
ChEBI: 4-sec-Butylphenol is an alkylbenzene.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes. To fight fire, use foam, CO2, dry chemical. Incompatible with oxidizing materials. See also PHENOL and other butyl phenols.
Potential Exposure
Butylphenols may be used as intermediates in manufacturing varnish and lacquer resins; as a germicidal agent in detergent disinfectants; as a pour point depressant, in motor-oil additives; de-emulsifier for oil; soap-antioxidant, plasticizer, fumigant, and insecticide
Shipping
UN2430 Alkylphenols, solid, n.o.s. (including C2-C12 homologues), Hazard class: 8; Labels: 8— Corrosive material
Incompatibilities
Vapors may form explosive mixture with air. These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may cause the gas to ignite and explode. Heat is also generated by the acid-base reaction with bases; such heating may initiate polymerization of the organic compound. React with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock
Properties of 4-(2-Butyl)phenol
| Melting point: | 46-59 °C(lit.) |
| Boiling point: | 135-136 °C25 mm Hg(lit.) |
| Density | 0.9860 |
| refractive index | 1.5182 |
| Flash point: | 240 °F |
| form | powder to crystal |
| pka | 10.11±0.26(Predicted) |
| color | White to Almost white |
| Water Solubility | 0.96g/L(25 ºC) |
| CAS DataBase Reference | 99-71-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-(1-methylpropyl)-(99-71-8) |
| EPA Substance Registry System | 4-sec-Butylphenol (99-71-8) |
Safety information for 4-(2-Butyl)phenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 4-(2-Butyl)phenol
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 99-71-8 4-sec-Butylphenol 99%View Details
99-71-8 4-sec-Butylphenol 99%View Details
99-71-8 -
 4-sec-Butylphenol CAS 99-71-8View Details
4-sec-Butylphenol CAS 99-71-8View Details
99-71-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
