3,4-Dihydroxyphenylacetic acid
Synonym(s):DOPAC;Homoprotocatechuic acid
- CAS NO.:102-32-9
- Empirical Formula: C8H8O4
- Molecular Weight: 168.15
- MDL number: MFCD00004338
- EINECS: 203-024-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
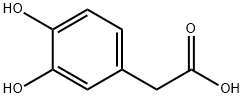
What is 3,4-Dihydroxyphenylacetic acid?
Description
3,4-Dihydroxyphenylacetic acid (DOPAC) is a metabolite of the neurotransmitter dopamine. Dopamine can be metabolized into one of three substances. One such substance is DOPAC. Another is 3-methoxytyramine (3-MT). Both of these substances are degraded to form homovanillic acid (HVA). Both degradations involve the enzymes monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT), albeit in reverse order: MAO catalyzes dopamine to DOPAC, and COMT catalyzes DOPAC to HVA; whereas COMT catalyzes dopamine to 3-MT and MAO catalyzes 3-MT to HVA. The third metabolic end-product of dopamine is norepinephrine (noradrenaline).
DOPAC can be oxidized by hydrogen peroxide, leading to the formation of toxic metabolites which destroy dopamine storage vesicles in the substantia nigra. This may contribute to the failure of levodopa treatment of Parkinson's disease. A MAO-B inhibitor such as selegiline or rasagiline can prevent this from happening.
wikipedia
Chemical properties
beige to light brown crystalline powder
The Uses of 3,4-Dihydroxyphenylacetic acid
3,4-Dihydroxyphenylacetic Acid is a metabolite of Dopamine (D533782).
The Uses of 3,4-Dihydroxyphenylacetic acid
3,4-Dihydroxyphenylacetic acid is an important metabolite when studying the behavior of the dopaminergic system.
Definition
ChEBI: (3,4-dihydroxyphenyl)acetic acid is a dihydroxyphenylacetic acid having the two hydroxy substituents located at the 3- and 4-positions. It is a metabolite of dopamine. It has a role as a human metabolite. It is a dihydroxyphenylacetic acid and a member of catechols. It is functionally related to a phenylacetic acid. It is a conjugate acid of a (3,4-dihydroxyphenyl)acetate.
General Description
3,4-dihydroxyphenylacetic acid (DOPAC) is a normal constituent of rat brain tissue. A mass fragmentographic method for determination of DOPAC in rat brain tissue has been described. It is major metabolite of dopamine (DA). The voltammetric reduction of DOPAC has been studied at a glassy carbon electrode modified with single-wall carbon nanotubes (SWNTs). Semi-automatic fluorometric assay technique for DOPAC has been reported.
Properties of 3,4-Dihydroxyphenylacetic acid
| Melting point: | 127-130 °C (lit.) |
| Boiling point: | 257.07°C (rough estimate) |
| Density | 1.3037 (rough estimate) |
| refractive index | 1.5090 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | water: soluble50mg/mL, clear, almost colorless |
| form | Powder, Crystals or Lumps |
| pka | 4.42±0.10(Predicted) |
| color | White to off-white |
| Water Solubility | Soluble in water and methanol. |
| BRN | 2211017 |
| InChI | InChI=1S/C8H8O4/c9-6-2-1-5(3-7(6)10)4-8(11)12/h1-3,9-10H,4H2,(H,11,12) |
| CAS DataBase Reference | 102-32-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, (3,4-dihydroxyphenyl)-,(102-32-9) |
| EPA Substance Registry System | 3,4-Dihydroxyphenylacetic acid (102-32-9) |
Safety information for 3,4-Dihydroxyphenylacetic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3,4-Dihydroxyphenylacetic acid
| InChIKey | CFFZDZCDUFSOFZ-UHFFFAOYSA-N |
| SMILES | C1(CC(O)=O)=CC=C(O)C(O)=C1 |
3,4-Dihydroxyphenylacetic acid manufacturer
BASR Fine Chemicals Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 102-32-9 3,4-DI HYDROXY PHENYL ACETIC ACID 98%View Details
102-32-9 3,4-DI HYDROXY PHENYL ACETIC ACID 98%View Details
102-32-9 -
 102-32-9 98%View Details
102-32-9 98%View Details
102-32-9 -
 3,4-Dihydroxyphenylacetic Acid CAS 102-32-9View Details
3,4-Dihydroxyphenylacetic Acid CAS 102-32-9View Details
102-32-9 -
 Homoprotocatechuic acid >98% (HPLC) CAS 102-32-9View Details
Homoprotocatechuic acid >98% (HPLC) CAS 102-32-9View Details
102-32-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1