3-Octanone
Synonym(s):Ethyl amyl ketone;Ethyl pentyl ketone
- CAS NO.:106-68-3
- Empirical Formula: C8H16O
- Molecular Weight: 128.21
- MDL number: MFCD00009515
- EINECS: 203-423-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
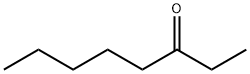
What is 3-Octanone?
Description
3-Octanone has a strong, penetrating, fruity odor reminiscent of lavender. It can be prepared by passing a mixture of vapors of caproic acid and acetic acid over ThO2 at 400°C, or by oxidation of d-ethyl n-amyl carbinol with chromates; another synthetic route is reported.
Description
3-Octanone, known to old-timers as n-amyl ethyl ketone, is a fragrant liquid produced by plants as diverse as lavender (Lavandula angustifolia), rosemary (Salvia rosmarinus), and nectarines (Prunus persica var. nucipersica). Its olfactory properties make it a useful flavor and fragrance agent.
But 3-octanone has a malicious side. A widely cultivated and consumed mushroom, Pleurotus ostreatus, produces the ketone to combat nematode (Caenorhabditis elegans) invasions. This distinct strategy kills the microscopic worms by disrupting their cell membranes and then uses them as nutrients.
In January this year, Yen-Ping Hsueh and several colleagues at Academica Sinica (Taipei, Taiwan?[Province of China]), National Taiwan University (Taipei), and other institutions in Taiwan and Japan demonstrated the mechanism that 3-octanone uses against the mushroom’s prey. When the researchers treated C. elegans directly with the ketone, it caused the same rapid paralysis, calcium influx, and neuronal cell death as does the carnivorous mushroom.
Chemical properties
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID
Chemical properties
3-Octanone has a strong, penetrating, fruity odor reminiscent of lavender.
Occurrence
Reported identified in the low-boiling fraction of the essential oil of lavender; also reported found in the essential oils of Lavandula vera (10%) and French lavender. Reported found in banana, bilberry, currants, guava, melon, blueberry, blackberry, strawberry jam, peas, fried potato, ginger, Mentha oils, thyme, cheeses, butter, fish, cooked meats, cognac, coffee, tea, roasted peanuts, pecan, soybean, olive, plum, beans, mushroom, wild marjoram, trassi, rice bran, litchi, calamus, buckwheat, rosemary, lemon balm, clary sage, rosemary, truffle, nectarine, anise hyssop and maté.
The Uses of 3-Octanone
3-Octanone can be produced by oxidation of 3-octanol or by heating propionic acid and caproic acid over thorium oxide. 3-Octanone is used as an ingredient in soaps, perfumes, lotions, and creams. It is also used as a flavoring agent in foods. U.S. production and importation of 3-octanone was estimated to be relatively low (,25,000 lb at a single site) in 2005 as data for 3-octanone were not included in the 2006 U.S. EPA Inventory Update Reporting database.
The Uses of 3-Octanone
Perfumery, solvent for nitrocellulose and vinyl resins.
The Uses of 3-Octanone
3-Octanone was used to study the effect of TiO2 photo catalyst on the rate and the ratio of products generated in photo catalytic oxidation of 3-octanone. It is used as a flavor and fragrance ingredient.
Preparation
By heating propionic and caproic acids over thorium oxide or by oxidation of ethyl amyl carbinol (3-octanol) (Arctander, 1969).
Definition
ChEBI: 3-octanone is a dialkyl ketone that is octane in which the two methylene protons at position 3 have been replaced by an oxo group. It has a role as a human urinary metabolite, an insect attractant, a fungal metabolite, an antifeedant, a plant metabolite and a biomarker. It derives from a hydride of an octane.
Aroma threshold values
Detection: 21 to 50 ppb
Taste threshold values
Taste characteristics at 10 ppm: mushroom, ketonic, cheesy and moldy with a fruity nuance.
Synthesis Reference(s)
Journal of the American Chemical Society, 97, p. 6863, 1975 DOI: 10.1021/ja00856a044
The Journal of Organic Chemistry, 32, p. 2356, 1967 DOI: 10.1021/jo01282a605
General Description
A clear colorless liquid with a pungent odor. Insoluble in water and partially soluble in alcohol. Flash point of 138°F. Vapors are denser than air and may have a narcotic effect in high concentrations. Used in making perfumes and as a solvent for nitrocellulose and vinyl resins.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Ketones, such as 3-Octanone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Hazard
Narcotic in high concentration. Moderate fire risk.
Health Hazard
May be harmful by inhalation, ingestion or skin absorption. Vapor or mist is irritating to eyes, mucous membrane and upper respiratory tract. Causes skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Vapor may travel considerable distance to a source of ignition and flash back.
Biochem/physiol Actions
Taste at 10 ppm
Safety Profile
Poison by intraperitoneal route. Moderately irritating to skin, eyes, and mucous membranes by inhalation. Narcotic in high concentration. Flammable liquid when exposed to heat, sparks, flame, or oxidizers. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke. See also KETONES.
Synthesis
It can be prepared by passing a mixture of vapors of caprioc acid and acetic acid over ThO2 at 400°C, or by oxidation of d-ethyl n-amyl carbinol with chromates; another synthetic route is reported.
Properties of 3-Octanone
| Melting point: | -23 °C |
| Boiling point: | 167-168 °C (lit.) |
| Density | 0.822 g/mL at 25 °C (lit.) |
| vapor pressure | 2.67hPa at 20℃ |
| refractive index | n |
| FEMA | 2803 | 3-OCTANONE |
| Flash point: | 115 °F |
| storage temp. | Flammables area |
| solubility | 2.60g/l |
| form | neat |
| appearance | colorless, oily liquid |
| color | Colorless to Light yellow |
| Odor | fruity odor |
| Water Solubility | 0.7 g/L |
| JECFA Number | 290 |
| Merck | 14,6753 |
| BRN | 1700021 |
| CAS DataBase Reference | 106-68-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Octanone(106-68-3) |
| EPA Substance Registry System | Ethyl amyl ketone (106-68-3) |
Safety information for 3-Octanone
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for 3-Octanone
3-Octanone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





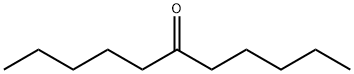
You may like
-
 3-Octanone 99%View Details
3-Octanone 99%View Details -
 106-68-3 99%View Details
106-68-3 99%View Details
106-68-3 -
 2-Chloropyridine CAS 106-68-3View Details
2-Chloropyridine CAS 106-68-3View Details
106-68-3 -
 3-Octanone CAS 106-68-3View Details
3-Octanone CAS 106-68-3View Details
106-68-3 -
 3-Octanone 106-68-3 98%View Details
3-Octanone 106-68-3 98%View Details
106-68-3 -
 3-Octanone 98%View Details
3-Octanone 98%View Details
106-68-3 -
 3-Octanone CAS 106-68-3View Details
3-Octanone CAS 106-68-3View Details
106-68-3 -
 3-Octanone CAS 106-68-3View Details
3-Octanone CAS 106-68-3View Details
106-68-3