3-METHYLOXINDOLE
Synonym(s):3-Methyloxindole
- CAS NO.:1504-06-9
- Empirical Formula: C9H9NO
- Molecular Weight: 147.17
- MDL number: MFCD04037370
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 14:36:51
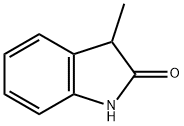
What is 3-METHYLOXINDOLE?
The Uses of 3-METHYLOXINDOLE
3-Methylindole metabolite.
The Uses of 3-METHYLOXINDOLE
• ;Reactant for enantioselective α-amination reactions1• ;Reactant for aldol reaction with glyoxal derivatives2• ;Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes3• ;Reactant for O-acetylation reactions4• ;Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions5
The Uses of 3-METHYLOXINDOLE
A versatile reactant. As 3-Methylindole metabolite. Reactant for enantioselective α-amination reactions, Reactant for aldol reaction with glyoxal derivatives, Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes, Reactant for O-acetylation reactions, Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions.
What are the applications of Application
3-Methyl-2-oxindole is a versatile reactant
Definition
ChEBI: 3-methyloxindole is a member of the class of oxindoles that is oxindole (1,3-dihydro-2H-indol-2-one) in which one of the hydrogens at position 3 has been replaced by a methyl group. It is a member of oxindoles and a methylindole.
General Description
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Properties of 3-METHYLOXINDOLE
| Melting point: | 117-121 °C(lit.) |
| Boiling point: | 279.3±29.0 °C(Predicted) |
| Density | 1.123±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| pka | 14.81±0.40(Predicted) |
| form | Solid |
| color | Light Beige to Beige |
| Water Solubility | Insoluble in water. |
| CAS DataBase Reference | 1504-06-9 |
Safety information for 3-METHYLOXINDOLE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 3-METHYLOXINDOLE
3-METHYLOXINDOLE manufacturer
GRK RESEARCH LABORATORIES PVT LTD
Khagga Life Sciences
CSR Laboratories Pvt Ltd
TRUE LABS
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 1504-06-9 98%View Details
1504-06-9 98%View Details
1504-06-9 -
 3-Methyl -2-indolinone 98%View Details
3-Methyl -2-indolinone 98%View Details
1504-06-9 -
 3-Methyl -2-indolinone 1504-06-9 99%View Details
3-Methyl -2-indolinone 1504-06-9 99%View Details
1504-06-9 -
 1504-06-9 3-Methyl-indol-2-one 98%+View Details
1504-06-9 3-Methyl-indol-2-one 98%+View Details
1504-06-9 -
 1504-06-9 3-Methylindolin-2-one 98%View Details
1504-06-9 3-Methylindolin-2-one 98%View Details
1504-06-9 -
 3-Methyl-2-oxindole CAS 1504-06-9View Details
3-Methyl-2-oxindole CAS 1504-06-9View Details
1504-06-9 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8