3-Chloro-1,2-propanediol
Synonym(s):α-Chlorohydrin;α-Glycerol chlorohydrin;α-Monochlorohydrin;3-MCPD
- CAS NO.:96-24-2
- Empirical Formula: C3H7ClO2
- Molecular Weight: 110.54
- MDL number: MFCD00004712
- EINECS: 202-492-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
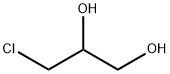
What is 3-Chloro-1,2-propanediol?
Chemical properties
Clear pale yellow liquid
The Uses of 3-Chloro-1,2-propanediol
(±)-3-Chloro-1,2-propanediol may be used as a reference standard for the determination of (±)-3-chloro-1,2-propanediol in food samples by gas chromatography with mass spectrometric detection (GC-MS).
The Uses of 3-Chloro-1,2-propanediol
A metabolite of Dichloropropanols
The Uses of 3-Chloro-1,2-propanediol
To lower the freezing point of dynamite; in the manufacture of dye intermediates. As rodent chemosterilant.
The Uses of 3-Chloro-1,2-propanediol
It is used in the synthesis of glycerol esters,amines, and other derivatives; to lower thefreezing point of dynamite; and as a rodentchemosterilant (Merck 1989).
Definition
ChEBI: A chloropropane-1,2-diol that is propane-1,2-diol substituted by a chloro group at position 3.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 1, p. 294, 1941
Synthetic Communications, 24, p. 1959, 1994 DOI: 10.1080/00397919408010203
Synthesis, p. 295, 1989
General Description
A colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Soluble in water. Hygroscopic.
Reactivity Profile
3-Chloro-1,2-propanediol is hygroscopic and may be sensitive to prolonged exposure to air. Glycols and their ethers undergo violent decomposition in contact with approximately 70% perchloric acid. .
Hazard
Toxic by ingestion, inhalation.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Health Hazard
Glycerol α-monochlorohydrin is a highlytoxic, teratogenic, and carcinogenic com pound. It is also an eye irritant.
Inhalation of 125 ppm in 4 hours wasfatal to rats. The lethal dose on mice viaintraperitoneal route was 10 mg/kg. Lowdosage can cause sleepiness, and on chronicexposure, weight loss
LD50 value, oral (rats): 26 mg/k
This compound is a strong teratomer,causing severe reproductive effects. Animal studies indicated that the adverse effects wereof spermatogenesis type, related to the testes,sperm duct, and Cowper’s gland. These werepaternal effects
Studies on rats indicated that high exposure levels to glycerol chlorohydrin can giverise to thyroid tumors.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. Experimental reproductive effects. A severe eye irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. A chemosterilant for rodents. Combustible when exposed to heat or flame. Reaction with perchloric acid forms a sensitive explosive product more powerful than glyceryl nitrate. When heated to decomposition it emits toxic fumes of Cl-.
Waste Disposal
Chemical incineration is the most appropriatemethod of disposal.
Properties of 3-Chloro-1,2-propanediol
| Melting point: | -40°C |
| Boiling point: | 213 °C(lit.) |
| Density | 1.322 g/mL at 25 °C(lit.) |
| vapor pressure | 0.04 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | Liquid |
| pka | 13.28±0.20(Predicted) |
| color | Clear pale yellow |
| Water Solubility | Soluble |
| FreezingPoint | -40℃ |
| Merck | 14,2145 |
| BRN | 635684 |
| CAS DataBase Reference | 96-24-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Propanediol, 3-chloro-(96-24-2) |
| IARC | 2B (Vol. 101) 2013 |
| EPA Substance Registry System | 3-Chloro-1,2-propanediol (96-24-2) |
Safety information for 3-Chloro-1,2-propanediol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H318:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Chloro-1,2-propanediol
3-Chloro-1,2-propanediol manufacturer
Trimax bio sciences private ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 3 Chloro-1,2-Propanediol 96-24-2 99%View Details
3 Chloro-1,2-Propanediol 96-24-2 99%View Details
96-24-2 -
 3-Chloro-1,2-propanediol 98%View Details
3-Chloro-1,2-propanediol 98%View Details -
 3-Chloro-1,2-propanediol 98%View Details
3-Chloro-1,2-propanediol 98%View Details
96-24-2 -
 α-Glycerol chlorohydrin CAS 96-24-2View Details
α-Glycerol chlorohydrin CAS 96-24-2View Details
96-24-2 -
 alpha-Chlorohydrine 98% (GC) CAS 96-24-2View Details
alpha-Chlorohydrine 98% (GC) CAS 96-24-2View Details
96-24-2 -
 3-Chloro-1,2-propanediol CAS 96-24-2View Details
3-Chloro-1,2-propanediol CAS 96-24-2View Details
96-24-2 -
 3-Chloro-1,2-propanediol CAS 96-24-2View Details
3-Chloro-1,2-propanediol CAS 96-24-2View Details
96-24-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1