3-Bromotoluene
- CAS NO.:591-17-3
- Empirical Formula: C7H7Br
- Molecular Weight: 171.03
- MDL number: MFCD00000085
- EINECS: 209-702-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
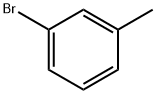
What is 3-Bromotoluene?
Chemical properties
colorless to light yellow liquid. Insoluble in water, soluble in ethanol and ether.
The Uses of 3-Bromotoluene
3-Bromotoluene was used in the precise determination of bromine isotope ratio in organic compounds by MC-ICPMS ( Multicollector-Inductively Coupled Plasma Mass Spectrometer). It undergoes palladium-catalyzed cyanation reaction in the presence of K4[Fe(CN)6] as the cyanide surrogate. It also undergoes palladium-catalyzed reaction with alkynyltriarylborates to yield trisubstituted alkenylboranes.
What are the applications of Application
3-Bromotoluene is used as a solvent (for fats, waxes or resins, and as a medium for carrying out reactions) and as a chemical intermediate.
Preparation
Synthesis of 3-Bromotoluene: It is derived from 3-bromo-4-aminotoluene by diazotization and reduction. The mixture of 95% ethanol, concentrated sulfuric acid and 3-bromo-4-aminotoluene was stirred and cooled to 10°C, and sodium nitrite solution was added to keep the reaction temperature not exceeding 10°C. After adding, continue stirring for 20min. Add the copper powder washed with ether, heat carefully, stop heating after the reaction starts, nitrogen will be released violently, and acetaldehyde will be formed. Then steam distillation is carried out until no oily substance evaporates. Separate the organic phase from the distillate and wash with 10% sodium hydroxide, water, concentrated sulfuric acid, and 5% sodium carbonate solution in sequence. Dry with anhydrous calcium chloride, filter, distill, collect 180-183 ℃ (99.75kPa) fractions to obtain colorless pure 3-bromotoluene with a yield of over 50%.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 1, p. 133, 1941
The Journal of Organic Chemistry, 57, p. 3772, 1992 DOI: 10.1021/jo00040a010
General Description
3-Bromotoluene is an electron-rich aryl bromide. It participates in the Heck reaction. 3-Bromotoluene undergoes palladium-catalyzed cyanation reaction in the presence of K4[Fe(CN)6] as the cyanide surrogate. It undergoes palladium-catalysed reaction with alkynyltriarylborates to yield trisubstituted alkenylboranes.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
3-Bromotoluene is incompatible with strong oxidizing agents.
Fire Hazard
3-Bromotoluene is combustible.
Properties of 3-Bromotoluene
| Melting point: | -40 °C |
| Boiling point: | 183.7 °C(lit.) |
| Density | 1.41 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 140 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.05g/l |
| form | Liquid |
| Specific Gravity | 1.410 |
| color | Clear colorless to light yellow |
| Water Solubility | insoluble |
| Merck | 14,1439 |
| BRN | 1903633 |
| Dielectric constant | 5.3600000000000003 |
| CAS DataBase Reference | 591-17-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-bromo-3-methyl-(591-17-3) |
| EPA Substance Registry System | m-Bromotoluene (591-17-3) |
Safety information for 3-Bromotoluene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H331:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 3-Bromotoluene
3-Bromotoluene manufacturer
Gravitas Pharma Pvt Ltd
JSK Chemicals
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

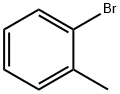

You may like
-
 3-Bromotoluene 98%View Details
3-Bromotoluene 98%View Details
591-17-3 -
 3-Bromo Toluene 591-17-3 98%View Details
3-Bromo Toluene 591-17-3 98%View Details
591-17-3 -
 3-Bromotoluene, 98% 591-17-3 99%View Details
3-Bromotoluene, 98% 591-17-3 99%View Details
591-17-3 -
 3-Bromotoluene CAS 591-17-3View Details
3-Bromotoluene CAS 591-17-3View Details
591-17-3 -
 3-Bromotoluene pure CAS 591-17-3View Details
3-Bromotoluene pure CAS 591-17-3View Details
591-17-3 -
 3-Bromotoluene CAS 591-17-3View Details
3-Bromotoluene CAS 591-17-3View Details
591-17-3 -
 3-Bromotoluene CAS 591-17-3View Details
3-Bromotoluene CAS 591-17-3View Details
591-17-3 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
