Propargyl bromide
Synonym(s):3-Bromo-1-propyne
- CAS NO.:106-96-7
- Empirical Formula: C3H3Br
- Molecular Weight: 118.96
- MDL number: MFCD00000241
- EINECS: 203-447-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
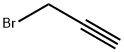
What is Propargyl bromide?
Description
Propargyl bromide is a Shock sensitive liquid, lachrymator. Supplied as an 80% solution in toluene stabilized with magnesium oxide. Alternative formulations are currently under development.
Chemical properties
yellow solution
The Uses of Propargyl bromide
Used to make propargylic amines employed in enyne metathesis.1Employed in the propargylation of spiro ketones,2 allylic alcohols,3 and enone complexes.4
The Uses of Propargyl bromide
Propargyl bromide, 80% in toluene is used to prepare betulonic acid-peptide conjugates with anti-inflammatory activity and propargylic amines employed in enyne metathesis. It finds application in the propargylation of spiro ketones, allylic alcohols and enone complexes. In Barbier-type reaction, it reacts with aldehydes to give alkyne alcohols. It is actively involved in the synthesis of polyethylene glycol (PEG) and peptide-grafted polyesters.
The Uses of Propargyl bromide
Propargyl Bromide is used in the synthesis of betulonic acid-peptide conjugates with anti-inflammatory activity. It is also used in the synthesis of PEG and peptide-grafted polyesters and make propargylic amines employed in enyne metathesis. Additionally, It is Employed in the propargylation of spiro ketones, allylic alcohols, and enone complexes.
What are the applications of Application
Propargyl bromide solution is an alkyne used for research purposes
Preparation
Add hydrogen bromide and solvent into the reaction flask, in the presence of CuBr and Cu catalyst, add propargyl alcohol dropwise with stirring, after the drop is completed, the temperature is slightly raised to react, and then post-processing, rectification to obtain bromopropane. alkyne. Reaction equation: CH≡C-CH2OH+HBr→CH≡C-CH2Br+H2O or the product can also be obtained by reacting propargyl alcohol with (PhO)3PBr2 in the presence of pyridine.
It is derived from the reaction of propargyl alcohol with phosphorus bromide. The propargyl alcohol was added to pyridine under cooling, and phosphorus tribromide was slowly added dropwise with stirring at -5°C, and the reaction temperature was maintained below 0°C. After the addition was completed, stirring was continued for 15 min. Then, vacuum distillation is performed to collect all the distillate, and fractional distillation is carried out again under normal pressure to obtain the finished product. Or add a small amount of pyridine to dry propargyl alcohol, add phosphorus tribromide and a small amount of pyridine solution dropwise at 0 °C under stirring, stir for 20 min after dropping, and distill under reduced pressure to obtain the product. Reaction equation: CH≡C-CH2OH+PBr3→CH≡C-CH2Br
Synthesis Reference(s)
Tetrahedron Letters, 4, p. 483, 1963 DOI: 10.1039/jr9630005127
General Description
A colorless to light yellow liquid substance with a sharp odor. Flash point 65°F. Denser than water and insoluble in water. Vapors are heavier than air. May be irritating to skin and eyes. Used to make other chemicals. 3-Bromopropyne may decompose explosively with mild shock.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
3-Bromopropyne is soluble in alcohol, ether, chloroform, carbon tetrachloride and carbon disulfide. 3-Bromopropyne is highly flammable and a dangerous fire risk, sensitive to shock. 3-Bromopropyne is used in organic syntheses, preparation of resins and perfume intermediates [Hawley]. There is a high danger of formation of explosive metal acetylides, when 3-Bromopropyne comes in contact with copper, high-copper alloys, mercury, or silver.
Hazard
Flammable, dangerous fire and explosion risk. Irritant.
Health Hazard
3-Bromopropyne is very toxic via the oral route. If inhaled, may be harmful; contact may cause burns to skin and eyes.
Fire Hazard
3-Bromopropyne detonates at 428F or more; ignites by impact. Emits highly toxic fumes of bromides when heated to decomposition. Reacts vigorously with oxidizing materials. Becomes shock-sensitive when mixed with chloropicrin. Unstable, avoid heat, flame, shock, and other chemicals
Pharmacology
Mode of action is considered to be reaction with nucleophiles in living organisms.
Safety Profile
A poison by ingestion. A dangerous fire hazard when exposed to heat or flame. The aerated liquid may be ignited by pressure. A dangerous, extremely shock-sensitive explosive. It can detonate when heated to 22O°C, by impact (especially when mixed with chloropicrin), or when heated whde confined. May explode on contact with copper, high copper alloys, mercury, or silver. Mixtures with trichloronitromethane are shockand heat- sensitive explosives. Can react vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of Br-. See also ACETYLENE COMPOUNDS and BROMIDES.
Metabolism
Reacts slowly with water to yield propargyl alcohol and bromide ion. It is expected to move as a typical fumigant through the soil. Probably not an ozone-depleting substance due to decomposition on absorption of ultraviolet light.
Properties of Propargyl bromide
| Melting point: | -61°C |
| Boiling point: | 97 °C |
| Density | 1.38 g/mL at 20 °C |
| refractive index | n |
| Flash point: | 65 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Methanol (Slightly) |
| form | Liquid |
| color | Colorless - Yellow |
| Water Solubility | Miscible with ethanol, ether, benzene, carbon tetrachloride and chloroform. Immiscible with water. |
| Sensitive | Light Sensitive |
| BRN | 605309 |
| Exposure limits | ACGIH: TWA 20 ppm OSHA: Ceiling 300 ppm; TWA 200 ppm NIOSH: IDLH 500 ppm; TWA 100 ppm(375 mg/m3); STEL 150 ppm(560 mg/m3) |
| CAS DataBase Reference | 106-96-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propyne, 3-bromo-(106-96-7) |
| EPA Substance Registry System | Propargyl bromide (106-96-7) |
Safety information for Propargyl bromide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H301:Acute toxicity,oral H304:Aspiration hazard H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propargyl bromide
| InChIKey | YORCIIVHUBAYBQ-UHFFFAOYSA-N |
Propargyl bromide manufacturer
SGT life sciences
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Propargyl bromide, stabilized with magnesium oxide CAS 106-96-7View Details
Propargyl bromide, stabilized with magnesium oxide CAS 106-96-7View Details
106-96-7 -
 Propargyl bromide 90-96% CAS 106-96-7View Details
Propargyl bromide 90-96% CAS 106-96-7View Details
106-96-7 -
 Propargyl bromide (80% wt.in toluene) contains 0.3% magnesium oxide as stabilizer CAS 106-96-7View Details
Propargyl bromide (80% wt.in toluene) contains 0.3% magnesium oxide as stabilizer CAS 106-96-7View Details
106-96-7 -
 Propargyl Bromide (106-96-7)View Details
Propargyl Bromide (106-96-7)View Details
106-96-7 -
 Propargyl Bromide Cas 106-96-7, 98%View Details
Propargyl Bromide Cas 106-96-7, 98%View Details
106-96-7 -
 Propargyl Bromide 80 In Toluene DensityView Details
Propargyl Bromide 80 In Toluene DensityView Details
106-96-7 -
 3-BromopropyneView Details
3-BromopropyneView Details
106-96-7 -
 3-Bromopropyne,View Details
3-Bromopropyne,View Details
106-96-7
