3-Aminocrotononitrile
Synonym(s):3-Amino-2-butenenitrile;3-Iminobutyronitrile;Diacetonitrile
- CAS NO.:1118-61-2
- Empirical Formula: C4H6N2
- Molecular Weight: 82.1
- MDL number: MFCD00008071
- EINECS: 214-266-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
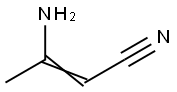
What is 3-Aminocrotononitrile?
Chemical properties
Yellowish flakes. 3-Amino-3-methylacrylonitrile [1118-61- 2], b-iminobutyronitrile, b-aminocrotononitrile, diacetonitrile, 3-amino-2-butenenitrile, NCCH=C (NH2) CH3, Mr 82.11, mp (cis-form) 79 – 83℃, mp (trans-form) 52 – 53℃, d774 0.9519, is a yellowish solid soluble in ethanol and diethyl ether; it is sparingly soluble in benzene and water.
The Uses of 3-Aminocrotononitrile
3-Aminocrotononitrile is used as intermediate for the syntheses of heterocycles (e.g. pyridines and pyrimidines) and for the production of polyurethane). It is used in the pharmaceutical (e.g. production of sulfsomizole) and dyestuff industries. Product Data Sheet
The Uses of 3-Aminocrotononitrile
3-Aminocrotononitrile reacts with ferrocenyl-1,2-enones to form ferrocenyl pyridines. It undergoes diazotization coupling reaction with p-substituted anilines to give 2-arylhydrazone-3-ketimino-butyronitriles.3-Aminocrotononitrile, (E)+(Z), is used in reaction with aryldiazonium salts gave, instead of the expected triazene, 2-arylhydrazono-3-ketobutyronitrile by electrophilic attack at the ?-carbon and hydrolysis of the resulting imine. It is also used as an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. 3-Aminocrotononitrile was used as bisnucleophilic reagent which on cyclocondensation with hexafluoroacetone(ethoxycarbonylimine) forms bis(trifluoromethyl)pyrimidinones.
Biochem/physiol Actions
3-Aminocrotononitrile reacts with ferrocenyl-1,2-enones to form ferrocenyl pyridines. It undergoes diazotization coupling reaction with p-substituted anilines to give 2-arylhydrazone-3-ketimino-butyronitriles.
Properties of 3-Aminocrotononitrile
| Melting point: | 79-81°C |
| Boiling point: | 120°C 4mm |
| Density | 0.952 |
| refractive index | 1.4449 (estimate) |
| Flash point: | 154°C |
| storage temp. | 2-8°C |
| solubility | 95% ethanol: soluble25mg/mL, clear, colorless to yellow |
| pka | 3.88±0.70(Predicted) |
| form | Flakes |
| color | Yellow |
| Water Solubility | Very soluble in water, soluble in ethanol. |
| BRN | 1719815 |
| CAS DataBase Reference | 1118-61-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butenenitrile, 3-amino-(1118-61-2) |
| EPA Substance Registry System | 2-Butenenitrile, 3-amino- (1118-61-2) |
Safety information for 3-Aminocrotononitrile
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 3-Aminocrotononitrile
3-Aminocrotononitrile manufacturer
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran




You may like
-
 1118-61-2 3-AMINOCROTONONITRILE 99%View Details
1118-61-2 3-AMINOCROTONONITRILE 99%View Details
1118-61-2 -
 1118-61-2 99%View Details
1118-61-2 99%View Details
1118-61-2 -
 1118-61-2 98%View Details
1118-61-2 98%View Details
1118-61-2 -
 3-Aminocrotononitrile, (E)+(Z) CAS 1118-61-2View Details
3-Aminocrotononitrile, (E)+(Z) CAS 1118-61-2View Details
1118-61-2 -
 3-Aminocrotononitrile CAS 1118-61-2View Details
3-Aminocrotononitrile CAS 1118-61-2View Details
1118-61-2 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
