3,6-Dichloro-1,2-benzenedithiol
- CAS NO.:87314-49-6
- Empirical Formula: C6H4Cl2S2
- Molecular Weight: 211.13
- MDL number: MFCD00216665
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-02-13 10:22:13
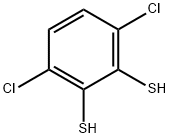
What is 3,6-Dichloro-1,2-benzenedithiol?
The Uses of 3,6-Dichloro-1,2-benzenedithiol
3,6-Dichloro-1,2-benzenedithiol is a stain/dye. Dyes and metabolites.
What are the applications of Application
3,6-Dichloro-1,2-benzenedithiol is a derivitized benzene compound
What are the applications of Application
3,6-Dichloro-1,2-benzenedithiol has been used as a ligand in thiolate complexes and a series of homo-chalcogenide and mixed-chalcogenide ligand complexes. For example, Direct reaction between Cu(ClO4)2 and 3,6-dichloro-1,2-benzenedithiol (HSC6H2Cl2SH) in the presence of basic salts leads to a series of bis(dithiolato)cuprate(III) [Cu(SC6H2Cl2S)2]? coordination complexes and coordination polymers. In addition, One-pot reactions between Ni(II), Pd(II), or Pt(II) salts and 3,6-dichloro-1,2-benzenedithiol (HSC6H2Cl2SH) in KOH medium under argon lead to a series of bis-dithiolene coordination polymer[1-2].
References
[1] Amo-Ochoa, Pilar et al. “Copper dithiolene [Cu(SC6H2Cl2S)2]? units connected to alkaline/copper complexes: from ionic assemblies to discrete molecular entities and coordination polymers?.” CrystEngComm 6 (2018): 957–963.
[2] Delgado, Esther et al. “Unprecedented layered coordination polymers of dithiolene group 10 metals: magnetic and electrical properties?.” Dalton Transactions 15 (2016): 6696–6701.
Properties of 3,6-Dichloro-1,2-benzenedithiol
| Melting point: | 58-60°C (lit.) |
| Boiling point: | 314.9±37.0 °C(Predicted) |
| Density | 1.523±0.06 g/cm3(Predicted) |
| pka | 4.40±0.50(Predicted) |
| InChI | InChI=1S/C6H4Cl2S2/c7-3-1-2-4(8)6(10)5(3)9/h1-2,9-10H |
Safety information for 3,6-Dichloro-1,2-benzenedithiol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H361:Reproductive toxicity H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3,6-Dichloro-1,2-benzenedithiol
| InChIKey | AJCUDWCLDWDLNY-UHFFFAOYSA-N |
| SMILES | C1(S)=C(Cl)C=CC(Cl)=C1S |
3,6-Dichloro-1,2-benzenedithiol manufacturer
ALTRAKEM PHARMA LIFE SCIENCES PRIVATE LIMITED
TRUE LABS
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 87314-49-6 3,6-Dichloro-1,2-benzenedithiol 99%View Details
87314-49-6 3,6-Dichloro-1,2-benzenedithiol 99%View Details
87314-49-6 -
 87314-49-6 3,6-dichlorobenzene-1,2-dithiol 98%View Details
87314-49-6 3,6-dichlorobenzene-1,2-dithiol 98%View Details
87314-49-6 -
 87314-49-6 99%View Details
87314-49-6 99%View Details
87314-49-6 -
 3,6-Dichloro-1,2-benzenedithiol 98%View Details
3,6-Dichloro-1,2-benzenedithiol 98%View Details
87314-49-6 -
 3,6-dichlorobenzene-1,2-dithiol 98%View Details
3,6-dichlorobenzene-1,2-dithiol 98%View Details
87314-49-6 -
 3,6-Dichloro-1,2-benzenedithiol CAS 87314-49-6View Details
3,6-Dichloro-1,2-benzenedithiol CAS 87314-49-6View Details
87314-49-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1