(2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE
- CAS NO.:144110-38-3
- Empirical Formula: C32H52N8O13S
- Molecular Weight: 788.87
- MDL number: MFCD07366491
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:00
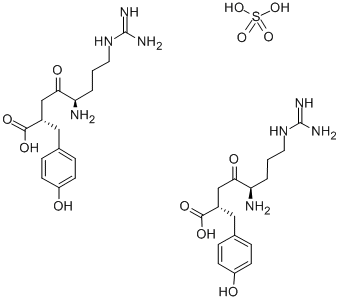
What is (2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE?
The Uses of (2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE
Arphamenine B Hemisulfate is a Zn2+-dependent exopeptidase that selectively removes arginine and/or lysine from the amino terminus of peptide substrates. This enzyme is a metalloprotease commonly found on the surface of mammalian cells, including macrophages and lymphocytes. Arphamenine B Hemisulfate is an aminopeptidase B inhibitor first isolated from bacteria. Like the aminopeptidase inhibitors bestatin and amastatin , arphamenine B enhances immune responses. Arphamenine B Hemisulfate is commonly used to characterize novel proteases.[Cayman Chemical]
What are the applications of Application
Arphamenine B is a specific aminopeptidase B inhibitor
Biological Activity
arphamenine b is a specific inhibitor of aminopeptidase b first isolated from bacteria [1]. aminopeptidase b (ap-b) is a zn2+-dependent exopeptidase which selectively removes arg and/or lys residues from the n terminus of several peptide substrates. aminopeptidase b has been involved in processing events occurring either during its intracellular transport along the secretory pathway or at the plasma membrane level [2].
in vitro
arphamenine b inhibited the activity of aminopeptidase enzyme with an ic50 value of 9.0 μm [2]. arphamenine b strongly inhibited transport by the oligopeptide/h+ symporter with the ec50 values of 15 to 67 μm. arphamenine at concentration 100 μm acted as either ineffective or weak inhibitor of membrane-associated hydrolysis [4]. arphamenine selectively suppressed dipeptide hydrolysis [4].
References
[1] umezawa h, aoyagi t, ohuchi s, et al. arphamenines a and b, new inhibitors of aminopeptidase b, produced by bacteria[j]. the journal of antibiotics, 1983, 36(11): 1572-1575.
[2] balogh a, cadel s, foulon t, et al. aminopeptidase b: a processing enzyme secreted and associated with the plasma membrane of rat pheochromocytoma (pc12) cells[j]. journal of cell science, 1998, 111(2): 161-169.
[3] sajid m, isaac r e, harrow i d. purification and properties of a membrane aminopeptidase from ascaris suum muscle that degrades neuropeptides af1 and af2[j]. molecular and biochemical parasitology, 1997, 89(2): 225-234.
[4] daniel h, adibi s a. functional separation of dipeptide transport and hydrolysis in kidney brush border membrane vesicles[j]. the faseb journal, 1994, 8(10): 753-759.
Properties of (2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE
| storage temp. | −20°C |
| solubility | ≤0.2mg/ml in DMSO |
| form | White to yellow powder |
Safety information for (2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE
Computed Descriptors for (2R,5S)-5-AMINO-8-GUANIDINO-4-OXO-2-P-HYDROXYPHENYLMETHYLOCTANOIC ACID HEMISULFATE MONOHYDRATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4