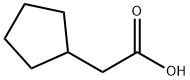
(2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
Synonym(s):α-Cyclopentyl-4-(2-quinolinylmethoxy)-(R)-benzeneacetic acid;(αR)-α-Cyclopentyl-4-(2-quinolinylmethoxy)-benzeneacetic acid;DG 031;Velifapon;Veliflapon
- CAS NO.:128253-31-6
- Empirical Formula: C23H23NO3
- Molecular Weight: 361.43
- MDL number: MFCD00870329
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
![(2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid Structural](https://img.chemicalbook.in/CAS/GIF/128253-31-6.gif)
What is (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid?
The Uses of (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
Prevention of acute cardiovascular events.
Biological Activity
bay-x 1005 is a selective inhibitor of 5-lipoxygenase-activating protein [1].5-lipoxygenase-activating protein (flap) is an integral protein and plays an important role in the activation of 5-lipoxygenase (5-lox) and the synthesis of leukotrienes, which regulating immune responses.bay-x 1005 is a selective inhibitor of leukotriene synthesis. bay x 1005 binds to flap and inhibits 5-lox translocation from the cytosol to membranes [1]. bay-x1005 inhibited ltb4 synthesis with ic50 values of 0.22, 0.026 and 0.039 μm for isolated pmnl of human, rat and mouse respectively and inhibited ltc4 synthesis with ic50 value of 0.021 μm in mouse macrophages [2].in the arachidonate-induced mouse ear inflammation test, bay-x 1005 inhibited myeloperoxidase activity and edema formation with ed50 values of 7.9 and 48.7, respectively [2].also, bay-x 1005 (100 mg/kg) reduced platelet-activating factor-induced death of mice by 51% in a dose-dependent way. in animal models, bay-x 1005 inhibited the synthesis of ltb4 and ltc4, which reduced edema formation, the vascular phenomena of inflammation and leukocyte immigration [3].
References
[1]. hatzelmann a, fruchtmann r, mohrs kh, et al. mode of action of the leukotriene synthesis (flap) inhibitor bay x 1005: implications for biological regulation of 5-lipoxygenase. agents actions, 1994, 43(1-2): 64-68.
[2]. müller-peddinghaus r, fruchtmann r, ahr hj, et al. bay x1005, a new selective inhibitor of leukotriene synthesis: pharmacology and pharmacokinetics. j lipid mediat, 1993, 6(1-3): 245-248.
[3]. müller-peddinghaus r, kohlsdorfer c, theisen-popp p, et al. bay x1005, a new inhibitor of leukotriene synthesis: in vivo inflammation pharmacology and pharmacokinetics. j pharmacol exp ther, 1993, 267(1): 51-57.
Properties of (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
| Melting point: | 169-171 °C |
| Boiling point: | 555.4±40.0 °C(Predicted) |
| Density | 1.242±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL (clear solution) |
| form | powder |
| pka | 4.47±0.10(Predicted) |
| color | white to beige |
Safety information for (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid](https://img.chemicalbook.in/CAS/GIF/128253-31-6.gif)

You may like
-
 BAY-X-1005 CAS 128253-31-6View Details
BAY-X-1005 CAS 128253-31-6View Details
128253-31-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1