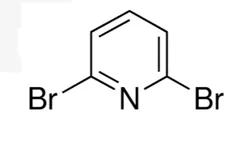
2,6-Dibromopyridine
- CAS NO.:626-05-1
- Empirical Formula: C5H3Br2N
- Molecular Weight: 236.89
- MDL number: MFCD00006223
- EINECS: 210-926-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-12 09:49:10
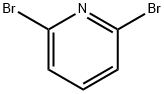
What is 2,6-Dibromopyridine?
Chemical properties
White to light yellow crystalline powder
The Uses of 2,6-Dibromopyridine
2,6-Dibromopyridine is used as a tridentate chelating ligand and in the formation of macrocycles containing the terpyridine moiety. It is also used to produce 6-bromo-2-methoxypyridine.
What are the applications of Application
2,6-Dibromopyridine is an important organic chemical reagent with a wide range of uses:
(1) Spectroscopic studies: There is a satisfactory correlation between the normal Raman spectra of 2,6-dibromopyridine in aqueous solution and the surface enhanced Raman (SER) spectra in silver-pure sols. In the SER spectra, the compounds are notable for the stretching of the vibrational modes of (py)CBr and (CC,CN)(py) in 2,6-dibromopyridine to give enhanced vibrational intensities at 1175 and 1369 cm-1 , respectively[1].
(2) Reaction reagent: Friedel–Crafts-type acylation of alkenes with acyl chlorides has been successfully conducted with a wide substrate scope by the combined use of AlCl3 and 2,6-dibromopyridine. Trisubstituted alkenes afford allylketones or vinylketones depending on the presence or absence of hydrogen atom(s) at the β-position to the acylation site, while monosubstituted alkenes exclusively afford vinylketones[2]. In addition, 2,6-Dibromopyridine can be brominated to form 2,4,6-tribromopyridine by reacting with a mixture of bromine at 450~ 550 °C[3]. It can also be lithiated with butyl lithium for the synthesis of L-739,010[4].
(3) A selective palladium-catalysed arylation of 2,6-dibromopyridine has been developed by employing N-heterocyclic carbene ligands. Selective mono-arylation was performed in water/acetonitrile solvent system at ambient temperature with catalyst loading of 0.1 mol%. This reaction was also found to proceed smoothly in water although at a slightly elevated temperature of 80 °C. 2,6-Disubstituted and diversely substituted 2,6-pyridines were also obtained in high yields which will be of importance to organic and medicinal chemists[5].
Purification Methods
Purify 2,6-dibromopyridine by steam distillation, then recrystallise it twice from EtOH. It does not form an HgCl2 salt. [den Hertog & Wibaut Recl Trav Chim Pays Bas 51 381 1932, Beilstein 20/5 V 435.]
References
[1] S. CHATTOPADHYAY S. K B. Surface-enhanced Raman spectroscopy of 2,5-dibromopyridine and 2,6-dibromopyridine[J]. Spectrochimica acta. Part A: Molecular spectroscopy, 1992. DOI:10.1016/0584-8539(92)80253-S.
[2] SHINYA TANAKA*. Acylation of Alkenes with the Aid of AlCl3 and 2,6-Dibromopyridine[J]. Organic Letters, 2019. DOI:10.1021/acs.orglett.9b02688.
[3] H. J. HERTOG C. R K ;W Combe. Substitution reactions in the pyridine nucleus at elevated temperatures (I). The bromination of 2,6‐dibromopyridine[J]. Recueil des Travaux Chimiques des Pays-Bas, 2010. DOI:10.1002/RECL.19580770109.
[4] D. CAI T. V D Hughes. A study of the lithiation of 2,6-dibromopyridine with butyllithium, and its application to synthesis of L-739,010[J]. Tetrahedron Letters, 1996. DOI:10.1016/0040-4039(96)00336-X.
[5] PRAJAPATI D, SCHULZKE C, KINDERMANN M K, et al. Selective palladium-catalysed arylation of 2,6-dibromopyridine using N-heterocyclic carbene ligands?[J]. RSC Advances, 2015. DOI:10.1039/C5RA10561G.
Properties of 2,6-Dibromopyridine
| Melting point: | 117-119 °C (lit.) |
| Boiling point: | 255°C |
| Density | 2.0383 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| Flash point: | 213 °C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | -3.65±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to gray or buff |
| Water Solubility | insoluble |
| BRN | 108922 |
| InChI | InChI=1S/C5H3Br2N/c6-4-2-1-3-5(7)8-4/h1-3H |
| CAS DataBase Reference | 626-05-1(CAS DataBase Reference) |
Safety information for 2,6-Dibromopyridine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,6-Dibromopyridine
| InChIKey | FEYDZHNIIMENOB-UHFFFAOYSA-N |
| SMILES | C1(Br)=NC(Br)=CC=C1 |
2,6-Dibromopyridine manufacturer
BTC pharm India
SAKEM LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 626-05-1 98%View Details
626-05-1 98%View Details
626-05-1 -
 2,6-Dibromopyridine 98%View Details
2,6-Dibromopyridine 98%View Details -
 2,6-Dibromopyridine CAS 626-05-1View Details
2,6-Dibromopyridine CAS 626-05-1View Details
626-05-1 -
 2,6-Dibromopyridine, 98% CAS 626-05-1View Details
2,6-Dibromopyridine, 98% CAS 626-05-1View Details
626-05-1 -
 Laboratory 2 6 DibromopyridineView Details
Laboratory 2 6 DibromopyridineView Details
626-05-1 -
 2 6 DibromopyridineView Details
2 6 DibromopyridineView Details
626-05-1 -
 2,6-DibromoPyridineView Details
2,6-DibromoPyridineView Details
626-05-1 -
 626-05-1 2,6-Dibromopyridine 98+View Details
626-05-1 2,6-Dibromopyridine 98+View Details
626-05-1
