2,5-Dimethoxybenzaldehyde
- CAS NO.:93-02-7
- Empirical Formula: C9H10O3
- Molecular Weight: 166.17
- MDL number: MFCD00003314
- EINECS: 202-211-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-26 15:35:00
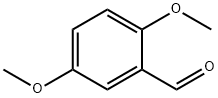
What is 2,5-Dimethoxybenzaldehyde?
Chemical properties
yellow crystalline solid
The Uses of 2,5-Dimethoxybenzaldehyde
2,5-Dimethoxybenzaldehyde is used in the preparation of 2,5-dimethoxyphenethylamine, which is utilized to prepare psychoactive drugs such as 2,5-dimethoxy-4-bromophenethylamine, 2,5-dimethoxy-4-iodophenethylamine and 4-Chloro-2,5-dimethoxy-phenethylamine. It acts as an intermediate in organic synthesis.
What are the applications of Application
2,5-Dimethoxybenzaldehyde can be used for:
(1) Raw material for organic synthesis, used in the preparation of 2,5-Dimethoxybenzeneethanamine, Dimethoxyamidochalcone, 4-(bromo or iodo)-2,5-Dimethoxybenzonitrile, 5-Hydroxy-2-Methoxybenzaldehyde, and other organic substances.
(2) Chemical reaction reagents for amination, bromination and cyclisation reactions.
What are the applications of Application
2,5-Dimethoxybenzaldehyde, also known as 2C-H, is an organic compound and a benzaldehyde derivative. It can be used to produce 2,5-dimethoxyphenethylamine. 2C-H is also used to produce many other substituted phenethylamines such as 2C-B, 2C-I and 2C-C.
Preparation
2,5-Dimethoxybenzaldehyde synthesis: Anethole is oxidized to anisaldehyde , which after isolation is subjected to a BaeyerVilliger oxidation reaction with performic or peracetic acid. The O-formyl-4-methoxyphenol obtained this way is hydrolyzed . 4-Methoxyphenol is subsequently formylated using the Reimer-Tiemann method and the obtained 2-hydroxy-5-methoxybenzaldehyde is methylated with dimethylsulfate to 2,5-dimethoxybenzaldehyde.
Reaction
A : Anisaldehyde from anethole via oxidative cleavage: 20 g anise oil was suspended in a mixture of 150 mL water and 30 mL conc. sulfuric acid; addition of 55 g sodium bichromate at such a rate that the temperature did not exceed 40°C. The reaction mixture was extracted with 4 x 125 mL toluene and the solvent evaporated. The residual oil was vacuum distilled to yield 9.1 g anisaldehyde.
B : O-formyl-4-methoxyphenol: 6 mL anisaldehyde was dissolved in 75 mL dichloromethane (DCM). A mixture of 12 g hydrogen peroxide and 10 mL conc. formic acid was added over 30 min. The reaction mixture was gently refluxed for 21 h.
C: B 4-methoxyphenol: Evaporating the solvent from reaction mixture and taking up the residue in 100 mL aqueous NaOH (20%) (25 mL MeOH as co-solvent) yielded 4.1 g 4-methoxyphenol as a white crystalline product after the usual work-up and purification steps.
D : Reimer-Tiemann formylation of 4-methoxyphenol: 124.1 g 4-methoxyphenol was dissolved in NaOH solution (320 g NaOH in 400 mL water). In total, 161 mL chloroform was added. The usual work-up and steam distillation yielded 109.8 g of a clear yellow oil that did not solidify upon standing at room temperature (GC/MS: 94% 2-hydroxy-5-methoxybenzaldehyde).
E: D Methylation of 2-hydroxy-5-methoxybenzaldehyde: The yellow oil from was used without further purification. A 250 mL RB flask was charged with 100 mL acetone, 14 g anhydrous potassium carbonate and 10 g 2-hydroxy-5-methoxybenzaldehyde; the mixture was brought at reflux temperature and 11 g dimethyl sulfate was added. The reaction was continued for 4 hours. The solvent is evaporated and the crude end product crystallized in cold water. Recrystallization from EtOH/water yielded 8.3 g 2,5-dimethoxybenzaldehyde (GC/MS: 98%+ 2,5-dimethoxybenzaldehyde)
Definition
ChEBI: 2,5-dimethoxy-Benzaldehyde is a dimethoxybenzene.
Hazard
2,5-Dimethoxybenzaldehyde is a strong skin and eye irritant. It caused drowsiness, euphoria and muscle weakness in oral lethal dose studies in mice.
Properties of 2,5-Dimethoxybenzaldehyde
| Melting point: | 46-48 °C (lit.) |
| Boiling point: | 146 °C/10 mmHg (lit.) |
| Density | 1.1708 (rough estimate) |
| refractive index | 1.5260 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 795mg/l |
| form | Crystalline Powder, Crystals and/or Chunks |
| color | Yellow to beige |
| Water Solubility | Soluble in chloroform and methanol. Slightly soluble in water. |
| Sensitive | Air Sensitive |
| BRN | 509301 |
| CAS DataBase Reference | 93-02-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde, 2,5-dimethoxy-(93-02-7) |
| EPA Substance Registry System | Benzaldehyde, 2,5-dimethoxy- (93-02-7) |
Safety information for 2,5-Dimethoxybenzaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,5-Dimethoxybenzaldehyde
| InChIKey | AFUKNJHPZAVHGQ-UHFFFAOYSA-N |
2,5-Dimethoxybenzaldehyde manufacturer
JSK Chemicals
Neugen Labs
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 93-02-7 2,5-DIMETHOXYBENZALDEHYDE 98%View Details
93-02-7 2,5-DIMETHOXYBENZALDEHYDE 98%View Details
93-02-7 -
 2,5-Dimethoxybenzaldehyde 98%View Details
2,5-Dimethoxybenzaldehyde 98%View Details -
 2,5-Dimethoxybenzaldehyde 98%View Details
2,5-Dimethoxybenzaldehyde 98%View Details
93-02-7 -
 2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
93-02-7 -
 2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
93-02-7 -
 2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
93-02-7 -
 2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
2,5-Dimethoxybenzaldehyde CAS 93-02-7View Details
93-02-7 -
 2,5 DIMETHOXYBENZALDEHYDEView Details
2,5 DIMETHOXYBENZALDEHYDEView Details
93-02-7
