2,4-Dichlorobenzyl alcohol
- CAS NO.:1777-82-8
- Empirical Formula: C7H6Cl2O
- Molecular Weight: 177.03
- MDL number: MFCD00004606
- EINECS: 217-210-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:44
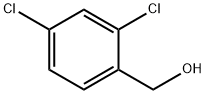
What is 2,4-Dichlorobenzyl alcohol?
Absorption
Dichlorobenzyl alcohol is released almost immediately from its formulation and reaches peak concentration after 3-4 minutes. The concentration in saliva after 120 minutes represents about 50% of the administered dose.
Toxicity
Fertility and mutagenicity studies do not indicate any effect driven by dichlorobenzyl alcohol. Overdose studies indicate that in systemic overdose there might be a presence of a CNS transitory stimulation followed by CNS and cardiovascular. Chronic administration is not recommended as it might alter the normal microbial balance of the throat. depression In rats, the reported LD50 is of about 2.7 g/kg when administered orally.
Chemical properties
white to light yellow crystal powde
The Uses of 2,4-Dichlorobenzyl alcohol
2,4-Dichlorobenzyl alcohol is an antiseptic found in commercially available throat lozenges. Also used deactivate Respiratory syncytial virus and SARS-Cov.
The Uses of 2,4-Dichlorobenzyl alcohol
An surgical antiseptic.
Background
Dichlorobenzyl alcohol is a mild antiseptic with a broad spectrum for bacterial and virus associated with mouth and throat infections. Dichlorobenzyl alcohol is considered as an active ingredient found in several marketed OTC products by Health Canada which has categorized this agent as an anatomical therapeutic chemical. On the other hand, dichlorobenzyl alcohol is categorized by the FDA in the inactive ingredient for approved drug products.
Indications
Dichlorobenzyl alcohol in combination with Amylmetacresol is available in over-the-counter products used for symptomatic relief of acute sore throat and postoperative sore throat.
What are the applications of Application
2,4-Dichlorobenzyl alcohol is an antiseptic
Definition
ChEBI: A member of the class of benzyl alcohols that is benzyl alcohol in which the hydrogens at positions 2 and 4 are replaced by chlorines.
General Description
2,4-Dichlorobenzyl alcohol is a constituent of commercially available lozenges for acute sore throat caused by upper respiratory tract infections.
Mechanism of action
In-vitro evidence has demonstrated the virucidal effect of Amylmetacresol (AMC) and 2,4-dichlorobenzyl alcohol (DCBA) on a number of viruses associated with the common cold; a reduction in viral load is believed to have benefits in reducing the symptoms. The local anesthetic action of AMC/DCBA throat lozenges, a combination of the potent channel blocker – AMC and the reduced potency for sodium channel blockade DCBA – attenuate the effects of AMC, possibly as a result of competitive binding, which acts on a sodium channel blocker, and may be effective in relieving symptoms due to inflammation. Therefore, AMC/DCBA throat lozenge is thought to represent a useful option to meet patients' needs and avoid unnecessary prescription of antibiotics[1].
Pharmacokinetics
In vitro studies with the combination of dichlorobenzyl alcohol and amylmetacresol have shown a virucidal against a number of viruses associated with the common cold which is observed by a reduction in the viral load. In clinical trials, administration of dichlorobenzyl alcohol lozenges has been shown to generate a reduced throat soreness and to provide pain relief and relief from difficulty in swallowing 5 minutes after administration. This effect can last for even 2 hours. The relief effect was shown to reach a steady-state after 45 minutes.
Clinical Use
Amylmetacresol and 2,4-dichlorobenzyl alcohol throat lozenges have been marketed in many countries worldwide for pain relief in acute sore throat. Amylmetacresol and 2,4-dichlorobenzyl alcohol throat lozenges have been shown to be safe and efficient in relieving of acute sore throat symptoms, and it produces an immediate symptomatic relief. Local symptomatic pain relief plays an important role in managing acute sore throat[1-2].
Side Effects
COMMON SIDE EFFECTS: Difficulty in breathing, Tongue soreness, Hypersensitivity reactions, Swelling of the face, Tongue, Throat and Neck.
Metabolism
Dichlorobenzyl alcohol is metabolized in the liver to form hippuric acid.
References
[1] Ting W Tan. “Effectiveness of amylmetacresol and 2,4-dichlorobenzyl alcohol throat lozenges in patients with acute sore throat due to upper respiratory tract infection: a systematic review protocol.” JBI database of systematic reviews and implementation reports (2017): 862–872.
[2] Thomas, Michael J. “ACP Journal Club. Amylmetacresol and 2, 4-dichlorobenzyl alcohol lozenges were better than placebo lozenges for relief of acute sore throat.” Annals of Internal Medicine 153 4 (2010): JC2-6.
Properties of 2,4-Dichlorobenzyl alcohol
| Melting point: | 55-58 °C(lit.) |
| Boiling point: | 150 °C (25 mmHg) |
| Density | 1.2970 (rough estimate) |
| vapor pressure | 0.154Pa at 25℃ |
| refractive index | 1.5756 (estimate) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | 1g/l |
| form | neat |
| pka | 13.60±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water, methanol and chloroform. |
| Merck | 14,3060 |
| BRN | 1448652 |
| CAS DataBase Reference | 1777-82-8(CAS DataBase Reference) |
| EPA Substance Registry System | 2,4-Dichlorobenzenemethanol (1777-82-8) |
Safety information for 2,4-Dichlorobenzyl alcohol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4-Dichlorobenzyl alcohol
| InChIKey | DBHODFSFBXJZNY-UHFFFAOYSA-N |
2,4-Dichlorobenzyl alcohol manufacturer
JSK Chemicals
Chemie Trade
Chemie Trade
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

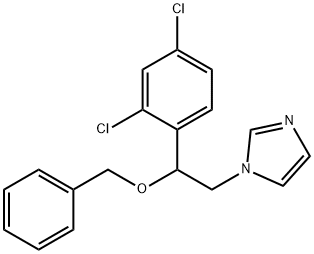
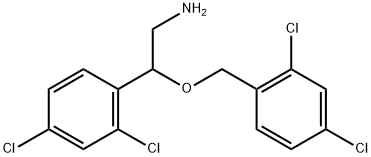
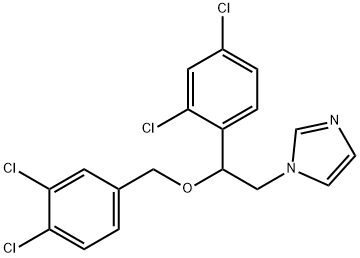
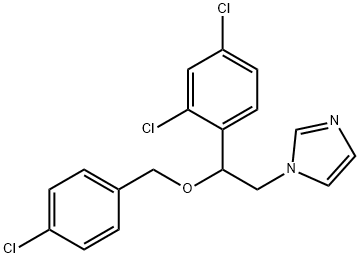
You may like
-
 1777-82-8 98%View Details
1777-82-8 98%View Details
1777-82-8 -
 2,4-Dichlorobenzyl alcohol 98%View Details
2,4-Dichlorobenzyl alcohol 98%View Details
1777-82-8 -
 1777-82-8 2,4-Dichlorobenzyl alcohol 99%View Details
1777-82-8 2,4-Dichlorobenzyl alcohol 99%View Details
1777-82-8 -
 2,4-Dichlorobenzyl Alcohol 1777-82-8 99%View Details
2,4-Dichlorobenzyl Alcohol 1777-82-8 99%View Details
1777-82-8 -
 2,4-Dichlorobenzyl Alcohol CAS 1777-82-8View Details
2,4-Dichlorobenzyl Alcohol CAS 1777-82-8View Details
1777-82-8 -
 2,4-Dichlorobenzyl alcohol CAS 1777-82-8View Details
2,4-Dichlorobenzyl alcohol CAS 1777-82-8View Details
1777-82-8 -
 2,4-Dichlorobenzyl alcohol CAS 1777-82-8View Details
2,4-Dichlorobenzyl alcohol CAS 1777-82-8View Details
1777-82-8 -
 2 4 Dichloro Benzyl AlcoholView Details
2 4 Dichloro Benzyl AlcoholView Details
1777-82-8
