2,2,2-TRIFLUOROETHYL VINYL ETHER
- CAS NO.:406-90-6
- Empirical Formula: C4H5F3O
- Molecular Weight: 126.08
- MDL number: MFCD00864407
- EINECS: 206-977-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
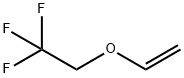
What is 2,2,2-TRIFLUOROETHYL VINYL ETHER?
Description
Fluroxene , 2,2,2-trifluoro-1- vinyloxyethane, is a colorless liquid with a pungent odor. It is inflammable and, at concentrations above 4 %, explosive in air. Fluroxene (MAC3.4) generally is used in a mixture with oxygen and nitrous oxide. Several cases of liver damage have been observed.
Description
Fluroxene, 2,2,2-trifluoro-1- vinyloxyethane, is a colorless liquid with a pungent odor. It is inflammable and, at concentrations above 4 %, explosive in air. Fluroxene (MAC3.4) generally is used in a mixture with oxygen and nitrous oxide. Several cases of liver damage have been observed.
Originator
Fluoromar, Ohio Medical, US ,1961
The Uses of 2,2,2-TRIFLUOROETHYL VINYL ETHER
Ca channel blocker, coronary vasodilator
Definition
ChEBI: Fluroxene is an organofluorine compound.
Manufacturing Process
The following process description is taken from US Patent 2,830,007.
270 grams 2,2,2-trifluoroethanol was added slowly to 15 grams of a cooled suspension of potassium metal in 250 ml of ethyl ether with stirring. When all the potassium metal had reacted, the resulting solution was fractionally distilled in order to remove the ethyl ether. The residue was placed in a bomb and the air was removed from the bomb by flushing with acetylene. The bomb was sealed and heated to 150°C. Acetylene was then introduced at 245 to 260 psi and the gas pressure was maintained for a period of 5 hours under mechanical agitation throughout the reaction. At the end of this time, heating was discontinued, the flow of acetylene was shut off and the bomb was allowed to cool to room temperature. The excess pressure in the bomb was reduced to atmospheric pressure by venting any gases through a dry ice cooled trap.
The reaction mixture comprising 2,2,2-trifluoroethyl vinyl ether, 2.2.2trifluoroethanol and potassium 2,2,2-trifluoroethylate was fractionally distilled, whereupon crude 2,2,2-trifluoroethyl vinyl ether was obtained which boiled at 42° to 45°C at 760 mm. More 2,2,2-trifluoroethyl vinyl ether was obtained when the distillation residue was returned to the bomb and reacted with acetylene in the same manner as hereinabove described.
The alkali metal hydroxides, instead of the alkali metals per se, can be employed to produce the alkali metal 2,2,2-trifluoroethanolate. However, this introduces water in the reaction mixture which requires removal prior to vinylation with acetylene. The crude products, on further distillation, yielded 2,2,2-trifluoroethyl vinyl ether having a boiling point of 43.1°C at 759 mm.
Therapeutic Function
Inhalation anesthetic
General Description
Volatile Liquid. Bp: 43°C. Density: 1.14 g cm-3 . Formerly used as an inhalation anesthetic.
Reactivity Profile
2,2,2-TRIFLUOROETHYL VINYL ETHER is non-flammable but combustible. Can form explosive mixtures with air. Reacts with strong oxidizing agents.
Safety Profile
Poison by inhalation. Moderately toxic by intraperitoneal route. An experimental teratogen. Human systemic effects by inhalation: jaundice and liver function tests impaired. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of F-. Used as an anesthetic. See also FLUORIDES and ETHERS.
Synthesis
The compound is prepared by catalytic vinylation
of 2,2,2-trifluoroethanol in the presence
of alkali-metal trifluoroethoxide :
Properties of 2,2,2-TRIFLUOROETHYL VINYL ETHER
| Boiling point: | 42-43°C |
| Density | 1,135 g/cm3 |
| refractive index | 1.3192 |
| Flash point: | -30°C |
| storage temp. | 2-8°C |
| form | A liquid |
| Water Solubility | 4mg/L(temperature not stated) |
| Merck | 14,4202 |
| BRN | 1745876 |
| Exposure limits | NIOSH: Ceiling 2 ppm(10.3 mg/m3) |
| CAS DataBase Reference | 406-90-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ethene, (2,2,2-trifluoroethoxy)- (406-90-6) |
Safety information for 2,2,2-TRIFLUOROETHYL VINYL ETHER
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H318:Serious eye damage/eye irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 2,2,2-TRIFLUOROETHYL VINYL ETHER
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![7-METHYL-2-(TRIFLUOROMETHYL)-4H,5H-PYRANO[4,3-B]PYRAN-4,5-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB5208443.gif)
![5-[4-(METHOXY)PHENYL]-2-(TRIFLUOROMETHYL)PYRAN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure9/GIF/CB6425559.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1