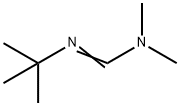
2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE
Synonym(s):BTMG
- CAS NO.:29166-72-1
- Empirical Formula: C9H21N3
- Molecular Weight: 171.28
- MDL number: MFCD01862972
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 17:06:38
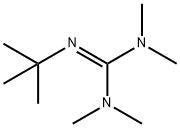
What is 2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE?
The Uses of 2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE
2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton′s base) is an excellent alternative to traditional inorganic bases for promoting the coupling reaction.
Preparation
To an ovendried, 500-mL, three-necked, round-bottomed flask, equipped with a nitrogen inlet with gas bubbler, magnetic stirring bar, thermometer, condenser, and a 250-mL dropping funnel, were added triphosgene (14.8 g, 0.05 mol) and anhydrous toluene (120 mL). The mixture was kept under argon and cooled to ca. 10 C° with the aid of an external ice bath. A solution of N,N,N,N-tetramethylurea (18.0 mL, 0.15 mol) in dry toluene (50 mL) was then slowly added over a period of 30 min. After completion of the addition, the mixture was allowed to warm to ambient temperature, and stirring was continued for a further 1 h. During this time, a white precipitate formed, consisting of the Vilsmeier salt. Then, tert-butylamine (47.3 mL, 0.45 mol) was slowly added to the mixture over a period of 30 min. After completion of the addition, the mixture was heated under reflux for 5 h and then cooled to room temperature. Anhydrous diethyl ether (200 mL) was added and the white precipitate was quickly removed by filtration. This precipitate had to be collected as quickly as possible to avoid hydrolysis to the starting urea. The precipitate turns pale-yellow if hydrolysis is occurring. In some instances, additional diethyl ether (300 mL) was needed to ensure complete transfer of the solids to the filtration apparatus. The precipitate was washed with a further quantity of anhydrous diethyl ether (300 mL) (the filtrate must be colorless, indicating that all impurities have been removed) and immediately dissolved in aqueous 25% sodium hydroxide solution (100 mL). The mixture was then extracted with diethyl ether (3 × 300 mL). The combined organic layers were dried (potassium carbonate), filtered, and the solvent was removed under reduced pressure. The resulting colorless liquid was purified by distillation (bp 88–89 C°/36 mmHg) to afford 18.7 g (73%) of 2-tertbutyl- 1,1,3,3-tetramethylguanidine 1779.
General Description
2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton′s base) is an excellent alternative to traditional inorganic bases for promoting the coupling reaction.
Properties of 2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE
| Boiling point: | 88-89 °C/43 mmHg (lit.) |
| Density | 0.85 |
| refractive index | n |
| Flash point: | 65 °C |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | clear liquid |
| pka | 13.81±0.70(Predicted) |
| color | Colorless to Light yellow |
| BRN | 2352408 |
Safety information for 2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-TERT-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE
| InChIKey | YQHJFPFNGVDEDT-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2-tert-Butyl-1,1,3,3-tetramethylguanidine CAS 29166-72-1View Details
2-tert-Butyl-1,1,3,3-tetramethylguanidine CAS 29166-72-1View Details
29166-72-1 -
 2-tert-Butyl-1,1,3,3-tetramethylguanidine CAS 29166-72-1View Details
2-tert-Butyl-1,1,3,3-tetramethylguanidine CAS 29166-72-1View Details
29166-72-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8