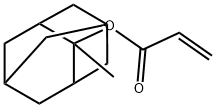
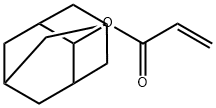
2-Methacryloyloxy-2-methyladamantane
- CAS NO.:177080-67-0
- Empirical Formula: C15H22O2
- Molecular Weight: 234.33
- MDL number: MFCD04114354
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-13 10:31:55
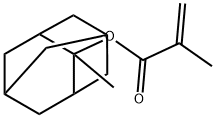
What is 2-Methacryloyloxy-2-methyladamantane?
Description
2-Methyl-2-adamantylmethacrylate is an aliphatic hydrocarbon with a trifluoromethyl group. It is a monomer that can be used to make polymers. 2-Methyl-2-adamantylmethacrylate has been shown to polymerize rapidly and form high molecular weight polymers. It reacts with hydroxyl groups to form ester linkages. This molecule also has the ability to self assemble, making it a supramolecular material. Impurities may affect the polymerization kinetics of this molecule and must be removed before use. As this compound is hydrophobic, it can only be patterned using a sublimable process.
Chemical properties
Off-white powder.
The Uses of 2-Methacryloyloxy-2-methyladamantane
2-Methyl-2-adamantylmethacrylate can be used to prepare Analog Semiconductors, Light-Emitting Diodes LED, Solar Photovoltaics PV.
General Description
Three different methacrylic monomers (g-butyrolactone methacrylate (GBLMA), 3-hydroxyl-1-adamantyl methacrylate (HAMA), and 2-methyl-2- adamantyl methacrylate (MAMA)) are the most often terpolymerized for 193 nm photoresists. The lactone group of GBLMA plays a vital role in the etching rate, facilitating binding to the substrate. The hydroxyl group of HAMA aids in the solubility and stability of the polymer. MAMA is particularly sensitive to 193 nm light and improves the etching resolution (chemical amplification)[1].
Synthesis
The synthesis of 2-Methyl-2-adamantylmethacrylate is as follows:Add 3546ml of n-heptane in the 5L four-necked flask equipped with stirring paddle, condenser tube and thermometer, turn on stirring, add 126.3g of 2-methyladamantanol to the system, and slowly add 0.022g polymerization inhibitor tetrachlorobenzoquinone to the reaction, add 110.8g basic ion exchange resin, adjust pH=7-8, cool down to -13°C, slowly add 110.8g vinyl methacrylate dropwise to the reaction system, keep the reaction for 7h, monitor the reaction progress, and take samples for detection After the reaction was completed, 1000 ml of water was added to the reaction system, and the mixture was stirred and extracted for phase separation. The organic phase was dried, concentrated under reduced pressure and evaporated to dryness to obtain 167 g of 2-methyl-2-adamantyl methacrylate product with a yield of 93.78%.

References
[1] M. Maríc. “Nitroxide‐mediated polymerization of adamantyl‐functional methacrylates for 193 nm photoresists.” Canadian Journal of Chemical Engineering 95 1 (2017): 708–716.
Properties of 2-Methacryloyloxy-2-methyladamantane
| Boiling point: | 301.3±11.0 °C(Predicted) |
| Density | 1.06±0.1 g/cm3(Predicted) |
| refractive index | 1.5050 to 1.5090 |
| Flash point: | 120°C(lit.) |
| storage temp. | 2-8°C |
| form | clear liquid |
| color | Colorless to Almost colorless |
| InChI | InChI=1S/C15H22O2/c1-9(2)14(16)17-15(3)12-5-10-4-11(7-12)8-13(15)6-10/h10-13H,1,4-8H2,2-3H3 |
Safety information for 2-Methacryloyloxy-2-methyladamantane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Methacryloyloxy-2-methyladamantane
| InChIKey | FDYDISGSYGFRJM-UHFFFAOYSA-N |
| SMILES | C(OC1(C)C2CC3CC1CC(C3)C2)(=O)C(C)=C |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 2-Methacryloyloxy-2-methyladamantane (stabilized with MEHQ) CAS 177080-67-0View Details
2-Methacryloyloxy-2-methyladamantane (stabilized with MEHQ) CAS 177080-67-0View Details
177080-67-0 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4