2-METHOXYESTRADIOL
Synonym(s):1,3,5(10)-Estratriene-2,3,17-triol 2-methyl ether;2,3,17β-Trihydroxy-1,3,5(10)-estratriene 2-methyl ether;2-Hydroxyestradiol 2-methyl ether;2-Methoxy-3,17β-dihydroxyestra-1,3,5(10)-triene;3,17β-Dihydroxy-2-methoxy-1,3,5(10)-estratriene
- CAS NO.:362-07-2
- Empirical Formula: C19H26O3
- Molecular Weight: 302.41
- MDL number: MFCD00010489
- EINECS: 263-807-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-27 16:59:26
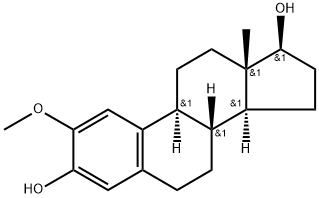
What is 2-METHOXYESTRADIOL?
Chemical properties
Off-White Solid
The Uses of 2-METHOXYESTRADIOL
2-Methoxyestradiol is a tubulin polymerization inhibitor and also decreases HIF-1 activity.
The Uses of 2-METHOXYESTRADIOL
A natural metabolite of 17?Estradiol which is devoid of estrogenic activity. Inhibits cell proliferation and angiogenesis. Binds to the colchicine binding site of tubulin, and has been suggested to function as a natural regulator of microtubule a
The Uses of 2-METHOXYESTRADIOL
A natural metabolite of 17β-Estradiol which is devoid of estrogenic activity. Inhibits cell proliferation and angiogenesis. Binds to the colchicine binding site of tubulin, and has been suggested to function as a natural regulator of microtubule assembly
The Uses of 2-METHOXYESTRADIOL
2-Methoxyestradiol depolymerizes microtubules and blocks HIF-1α nuclear accumulation and HIF-transcriptional activity. Phase 2.
The Uses of 2-METHOXYESTRADIOL
2-Methoxy 17β-Estradiol is a natural metabolite of 17β-Estradiol which is devoid of estrogenic activity. Inhibits cell proliferation and angiogenesis. 2-Methoxy 17β-Estradiol binds to the colchicine binding site of tubulin, and has been suggested to function as a natural regulator of microtubule assembly and function.
What are the applications of Application
2-Methoxyestradiol is a disruptor of microtubule function and HIF-1 inhibitor
Definition
ChEBI: A 17beta-hydroxy steroid, being 17beta-estradiol methoxylated at C-2.
Biological Activity
Apoptotic, antiproliferative and antiangiogenic agent, in vitro and in vivo ; acts via an estrogen receptor-independent mechanism. Induces p53-induced apoptosis via two pathways: activation of p38 and NF- κ B; and activation of JNK and AP-1 leading to Bcl-2 phosphorylation. Also upregulates death receptor 5 and binds to tubulin, inhibiting its assembly.
Properties of 2-METHOXYESTRADIOL
| Melting point: | 188-190°C |
| Boiling point: | 464.4±45.0 °C(Predicted) |
| Density | 1.178±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO: 10 mg/mL |
| form | crystalline |
| pka | 10.29±0.60(Predicted) |
| color | light yellow |
| λmax | 286nm(EtOH)(lit.) |
| CAS DataBase Reference | 362-07-2(CAS DataBase Reference) |
Safety information for 2-METHOXYESTRADIOL
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H350:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-METHOXYESTRADIOL
| InChIKey | CQOQDQWUFQDJMK-SSTWWWIQSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

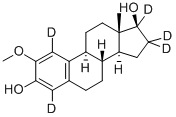
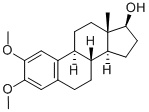
![2-METHOXYESTRADIOL [6,7-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB9703966.gif)




You may like
-
 2-Methoxy-β-estradiol CAS 362-07-2View Details
2-Methoxy-β-estradiol CAS 362-07-2View Details
362-07-2 -
 2-Methoxyestradiol CAS 362-07-2View Details
2-Methoxyestradiol CAS 362-07-2View Details
362-07-2 -
 2-Methoxyestradiol CAS 362-07-2View Details
2-Methoxyestradiol CAS 362-07-2View Details
362-07-2 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0