2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
- CAS NO.:583-91-5
- Empirical Formula: C5H10O3S
- Molecular Weight: 150.2
- MDL number: MFCD00070490
- EINECS: 209-523-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 09:56:27
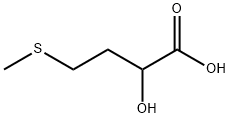
What is 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID?
The Uses of 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
2-Hydroxy-4-(methylthio)butyric Acid is used to improve stem activity to eliminate deoxynivalenol-induced intestinal injury.
The Uses of 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
Methionine hydroxy analogue
What are the applications of Application
DL-2-Hydroxy-4-(methylthio)butanoic Acid-13C4 is a Methionine hydroxy analogue
Definition
ChEBI: 2-hydroxy-4-(methylthio)butanoic acid is a thia fatty acid.
General Description
Light brown liquid.
Reactivity Profile
An organosulfide, alcohol, and organic acid. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt.
Health Hazard
Corrosive to the eyes and moderately irritating to the skin.
Properties of 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
| Boiling point: | 74°C |
| Density | 1.277±0.06 g/cm3 | Condition: Temp: 20 °C Press: 760 Torr |
| refractive index | 1.5151 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in DMSO:0.0(Max Conc. mg/mL);0.0(Max Conc. mM) |
| pka | 3.67±0.10(Predicted) |
| Merck | 14,5976 |
| CAS DataBase Reference | 583-91-5(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Hydroxy-4-(methylthio) butanoic acid (583-91-5) |
Safety information for 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
Computed Descriptors for 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID
| InChIKey | ONFOSYPQQXJWGS-UHFFFAOYSA-N |
2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID manufacturer
Clickchem Research LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![DL-2-HYDROXY-4-(METHYL THIO) BUTYRIC ACID, [1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB4486181.gif)

![Bis[2-hydroxy-4-(methylthio)butyric acid]calcium salt,2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID CALCIUM SALT](https://img.chemicalbook.in/CAS/GIF/4857-44-7.gif)
![Bis[(R)-2-hydroxy-4-(methylthio)butyric acid]calcium salt](https://img.chemicalbook.in/CAS/GIF/71597-86-9.gif)
![Bis[(S)-2-hydroxy-4-(methylthio)butyric acid]calcium salt](https://img.chemicalbook.in/CAS/GIF/71597-87-0.gif)

You may like
-
 2-Hydroxy-4-(methylthio)butyric acid, 85% CAS 583-91-5View Details
2-Hydroxy-4-(methylthio)butyric acid, 85% CAS 583-91-5View Details
583-91-5 -
 2-Hydroxy-4-(methylthio)butyric Acid (65-72% in Water) CAS 583-91-5View Details
2-Hydroxy-4-(methylthio)butyric Acid (65-72% in Water) CAS 583-91-5View Details
583-91-5 -
 2-HYDROXY-4-(METHYLSUFINYL)BUTANOIC ACID 90 % AboveView Details
2-HYDROXY-4-(METHYLSUFINYL)BUTANOIC ACID 90 % AboveView Details
583-91-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
