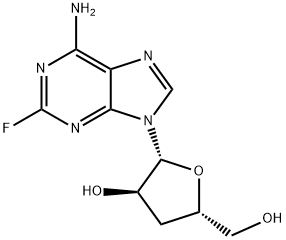
2-Fluoroadenosine
- CAS NO.:146-78-1
- Empirical Formula: C10H12FN5O4
- Molecular Weight: 285.23
- MDL number: MFCD00866394
- EINECS: 633-923-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
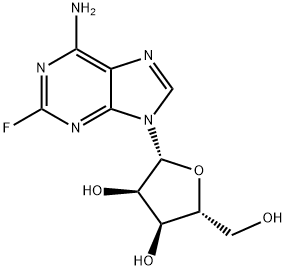
What is 2-Fluoroadenosine?
Description
2-Fluoroadenosine (F-Ado) was developed at Southern Research Institute in 1957 as a potential anticancer drug. 2-Fluoroadenosine is not deaminated by adenosine deaminase but metabolized to triphosphate as shown in vitro. The drug was also shown to be a potent inhibitor of lymphocyte-mediated cytolysis.
The Uses of 2-Fluoroadenosine
2-Fluoroadenosine is a fluorinated analog of Adenoside nucleotide. It is used as an intermediate for the drug fludarabine. Fludarabine is a purine analogue and antineoplastic agent. It is a chemotherapy medication used in the treatment of leukemia and lymphoma.
Definition
ChEBI: 2-fluoroadenosine is a member of adenosines and an organofluorine compound.
Biological Functions
2-Fluoroadenosine (F-Ado) was synthesized by Montgomery and his co-workers. This compound has been found to suppress the growth of H.Ep. No. 2 cells growing in culture. Bennett and Smithers have shown that in the same cells, the synthesis of 5-phosphoribosylamine was markedly inhibited by both 2- fluoroadenine and F-Ado. The compound was found to inhibit the incorporation of variously labeled precursors into ribonucleic acid in whole Ehrlich ascites cells. Upon incubation with ascites cells, the nucleoside was readily converted to the 5’-mono-, 5’-di-, and 5’-triphosphates.In addition, striking potentiation of the inhibition of human platelet aggregation by combinations of forskolin with PGE 1 or F-Ado [1-2].
Synthesis
A novel method for the introduction of fluorine in the purine 2-position is described and employed in the synthesis of a potential antimycobacterial compound. Also 2-fluoroadenosine has been synthesized for the first time from adenosine with perbenzoylated 2-nitroadenosine as a key intermediate. Mild reaction conditions are employed and few synthetic steps are required. The novel synthesis of fluoroadenosine can be regarded as a formal synthesis of the antileukemic drug fludarabine phosphate.
[1] MORTEN BR?NDVANG L. G. A Novel Method for the Introduction of Fluorine into the Purine 2-Position: Synthesis of 2-Fluoroadenosine and a Formal Synthesis of the Antileukemic Drug Fludarabine[J]. Synthesis-Stuttgart, 2006, 87 1: 2993-2995. DOI:10.1055/S-2006-942544.
References
[1] H T Shigeura. “Metabolism of 2-fluoroadenosine by Ehrlich ascites cells.” Archives of biochemistry and biophysics 111 3 (1965): 713–9.
[2] Kailash C. Agarwal, Robert E. Parks Jr. “Synergistic inhibition of platelet aggregation by forskolin plus PGE1 or 2-fluoroadenosine: Effects 2′,5′-dideoxyadenosine and 5′-methylthioadenosine.” Biochemical pharmacology 31 22 (1982): Pages 3713-3716.
Properties of 2-Fluoroadenosine
| Melting point: | 240 °C (D)(lit.) |
| Boiling point: | 747.3±70.0 °C(Predicted) |
| Density | 2.17±0.1 g/cm3(Predicted) |
| refractive index | -72 ° (C=0.1, EtOH) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly, Sonicated), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 13.05±0.70(Predicted) |
| color | Off-White |
| InChI | InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 |
| CAS DataBase Reference | 146-78-1(CAS DataBase Reference) |
Safety information for 2-Fluoroadenosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Fluoroadenosine
| InChIKey | HBUBKKRHXORPQB-UUOKFMHZSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C3C(=C(N=C(F)N=3)N)N=C2)[C@H](O)[C@@H]1O |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 2-Fluoroadenosine CAS 146-78-1View Details
2-Fluoroadenosine CAS 146-78-1View Details
146-78-1 -
 2-Fluoroadenosine CAS 146-78-1View Details
2-Fluoroadenosine CAS 146-78-1View Details
146-78-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4