2-Ethylhexanol
Synonym(s):2-Ethyl-1-hexanol;Isocotyl alcohol, Isooctanol;Isooctyl alcohol
- CAS NO.:104-76-7
- Empirical Formula: C8H18O
- Molecular Weight: 130.23
- MDL number: MFCD00004746
- EINECS: 203-234-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:24
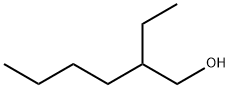
What is 2-Ethylhexanol?
Chemical properties
2-Ethylhexanol is a clear, colorless to pale yellow oily liquid. It has a mild, oily, sweet, slightly floral odor reminiscent of rose and sweet, fatty-floral taste with a fruity note. Soluble in 720 times water, miscible in most organic solvents.
Occurrence
Reported found in papaya, peach, pear, blackberry, strawberry, cabbage, Parmesan and mozzarella cheese, butter, roasted chicken, cognac, sherry, grape wines, tea, avocado, kiwifruit, crab and clam.
The Uses of 2-Ethylhexanol
2-Ethylhexanol is the most important C8 alcohol and is mainly used as the alcohol component for the manufacture of ester plasticizers for soft poly(vinyl chloride) (PVC). Other minor uses include the manufacturing of 2-ethylhexyl acrylate, as a dispersing agent and wetting agent, as a solvent for gums and resins, as a cosolvent for nitrocellulose, and in ceramics, paper coatings, rubber latex, textiles, and fragrances.
The Uses of 2-Ethylhexanol
2-Ethyl-1-hexanol is used as a flavor, fragrance and plasticizer. It is used to prepare diesters bis(2-ethylhexyl) phthalate. It reacts with nitric acid and used as an octane booster. Its ester, 2-ethylhexyl ester is a component of sunscreen octocrylene. Further, it is used as a low volatility solvent for resins, animal fats, waxes, vegetable oils and petroleum derivatives. In addition to this, it is used in plasticizer, dioctyl phthalate, which is used in the production of polyvinyl chloride products.
Definition
ChEBI: 2-Ethylhexanol is a primary alcohol that is hexan-1-ol substituted by an ethyl group at position 2. It has a role as a volatile oil component and a plant metabolite.
What are the applications of Application
2-Ethyl-1-hexanol is suitable for use in a study to compare its susceptibilities of dynamic heat capacity and dielectric polarization under isothermal conditions. It may be used to study lipase-catalyzed transesterification (alcoholysis) of rapeseed oil and 2-ethyl-1-hexanol in the absence of solvent. 2-Ethyl-1-hexanol may be used in broadband dielectric spectroscopy studies of the polyalcohols-glycerol, xylitol and sorbitol. It may be used in the preparation of porous beads.
Preparation
2-ethylhexanol synthesis: Condensation of acetaldehyde into butanol aldehyde, dehydration to obtain crotonaldehyde, hydrogenation to n-butyraldehyde, condensation dehydration to obtain 2-ethyl-2-hexenal, and then hydrogenation to obtain 2-ethylhexanol.
General Description
2-ethyl hexanol appears as a dark brown liquid with an aromatic odor. Insoluble in water and less dense than water. Flash point between 140 - 175°F. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Ethylhexanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. 2-Ethylhexanol is incompatible with strong oxidizing agents and strong acids.
Health Hazard
Anesthesia, nausea, headache, dizziness; mildly irritating to skin and eyes.
Fire Hazard
2-Ethylhexanol is combustible.
Safety Profile
Moderately toxic by ingestion, skin contact, intraperitoneal, subcutaneous, and parented routes. An experimental teratogen. Other experimental reproductive effects. A severe eye and moderate skin irritant. Mutation data reported. A dangerous fire hazard when ex posed to heat or flame; can react vigorously with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ALCOHOLS.
Synthesis
By hydrogenation of aldehydes obtained by the oxo process; also synthesized from propylene; by catalytic reduction of 2-ethyl-2-hexenal and other similar patented processes.
Carcinogenicity
Male and female F344 rats were dosed by oral gavage with 0, 50, 150, or 500 mg/kg 2-ethylhexanol (0.005% in aqueous Cremophor EL, a polyoxyl 35 castor oil), 5 days/week for 2 years. There were no differences of biological importance between the vehicle control and a water control group that was included in the study. There was no evidence of treatment-related neoplastic lesions in any of the exposed groups.
Properties of 2-Ethylhexanol
| Melting point: | −76 °C(lit.) |
| Boiling point: | 183-186 °C(lit.) |
| Density | 0.833 g/mL at 25 °C(lit.) |
| vapor density | 4.49 (vs air) |
| vapor pressure | 0.2 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3151 | 2-ETHYL-1-HEXANOL |
| Flash point: | 171 °F |
| storage temp. | Store below +30°C. |
| solubility | Water |
| form | Liquid |
| pka | 15.05±0.10(Predicted) |
| color | Clear |
| Odor | sweet. |
| PH | 7 (1g/l, H2O, 20℃) |
| explosive limit | 0.88%, 104°F |
| Odor Threshold | 0.013ppm |
| Water Solubility | 1 g/L (20 ºC) |
| Merck | 14,3808 |
| JECFA Number | 267 |
| BRN | 1719280 |
| Dielectric constant | 3.4(18℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 104-76-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Hexanol, 2-ethyl-(104-76-7) |
| EPA Substance Registry System | 2-Ethylhexanol (104-76-7) |
Safety information for 2-Ethylhexanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Ethylhexanol
| InChIKey | YIWUKEYIRIRTPP-UHFFFAOYSA-N |
2-Ethylhexanol manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 2 Ethyl Hexanol (EHA) 98%View Details
2 Ethyl Hexanol (EHA) 98%View Details -
 2 Ethyl Hexanol 99%View Details
2 Ethyl Hexanol 99%View Details -
 2-Ethylhexanol CASView Details
2-Ethylhexanol CASView Details -
 2 Ethyl HexanolView Details
2 Ethyl HexanolView Details
104-76-7 -
 2- EthylhexanolView Details
2- EthylhexanolView Details
104-76-7 -
 2 Ethyl HexanolView Details
2 Ethyl HexanolView Details
104-76-7 -
 Octanol 2 Ethyl HexanolView Details
Octanol 2 Ethyl HexanolView Details
104-76-7 -
 Industrial Grade 2 Ethyl Hexanol, >99%View Details
Industrial Grade 2 Ethyl Hexanol, >99%View Details
104-76-7
