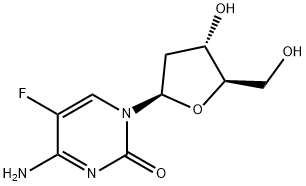
2'-DEOXY-5-FLUOROCYTIDINE
Synonym(s):2′-deoxy-5-fluoro-Cytidine;FCDR;FdCyd
- CAS NO.:10356-76-0
- Empirical Formula: C9H12FN3O4
- Molecular Weight: 245.21
- MDL number: MFCD00077348
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-05 19:05:58
What is 2'-DEOXY-5-FLUOROCYTIDINE?
Description
5-Fluoro-2-Deoxycytidine is an antimetabolite consisting of a fluorinated pyrimidine analog with potential antineoplastic activity. As a prodrug, 5-fluoro-2-deoxycytidine is converted by intracellular deaminases to the cytotoxic agent 5-Fluorouracil (5-FU). 5-FU is subsequently metabolized to active metabolites including 5-fluoro-2-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). FdUMP binds to and inhibits thymidylate synthase, thereby reducing the production of thymidine monophosphate, which leads to depletion of thymidine triphosphate. This inhibits DNA synthesis and cell division. FUTP competes with uridine triphosphate for incorporation into the RNA strand thus leading to an inhibition of RNA and protein synthesis. Other fluorouracil metabolites also get incorporated into both DNA and RNA, thereby further hampering cellular growth.
The Uses of 2'-DEOXY-5-FLUOROCYTIDINE
5-fluoro-2'-deoxycytidine has been used in trials studying the treatment of Neoplasms, Lung Neoplasms, Breast Neoplasms, Head and Neck Neoplasms, and Urinary Bladder Neoplasms, among others.
The Uses of 2'-DEOXY-5-FLUOROCYTIDINE
5-Fluoro-2''-deoxycytidine is a mechanism-based DNMT inhibitor.
What are the applications of Application
5-Fluoro-2′-deoxycytidine is a mechanism-based DNMT inhibitor
General Description
Fine white powder.
Air & Water Reactions
2'-DEOXY-5-FLUOROCYTIDINE is probably light and air sensitive.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 2'-DEOXY-5-FLUOROCYTIDINE emits toxic fumes of fluoride ion and NOx.
Fire Hazard
Flash point data for 2'-DEOXY-5-FLUOROCYTIDINE are not available. 2'-DEOXY-5-FLUOROCYTIDINE is probably combustible.
Pharmacology
5-Fluoro-2'-deoxycytidine (FdCyd), a fluoropyrimidine nucleoside analog, has a short (10-minute) half-life and is rapidly degraded in vivo by cytidine deaminase. However, coadministration with tetrahydrouridine (THU), an inhibitor of cytidine/deoxycytidine deaminase, has been shown to increase the AUC of the parent compound more than 4-fold. Increased FdCyd exposure allows it to be taken up intracellularly and converted to its triphosphate, which is incorporated into DNA and inhibits the action of the enzyme DNA methyltransferase (DNMT). Inhibition of DNMT, and in turn DNA methylation, can result in the re-expression of tumor suppressor genes.
Primary objective:
-Determine progression-free survival (PFS) and/or the response rate (CR + PR) of FdCyd administered 5 days per week for 2 weeks, in 28-day cycles, by intravenous infusion over 3 hours along with THU in patients with breast cancer, head and neck cancer, non-small cell lung cancer, and urothelial transitional cell carcinoma.
Exploratory objectives:
Evaluate whether treatment with FdCyd and THU alters DNA methylation patterns in tumor biopsy samples before and during treatment by LINE-1 analysis.
Evaluate the safety and tolerability of FdCyd (100 mg/m(2)) + THU (350 mg/m(2)) administered 5 days per week for 2 weeks, in 28-day cycles, by intravenous infusion over 3 hours.
Measure changes in the number of CTCs following treatment with FdCyd plus THU.
Clinical Use
A DNA methyltransferase inhibitor currently in clinical trials for breast cancer and other solid tumors. Like 5’-Azacytidine and decitabine, 2’-Deoxy-5-fluorocytidine is a pyrimidine analog that integrates into chromatin to inhibit DNA methylation. It blocks proliferation in colon cancer-derived HCT116 cells by activating DNA response pathways. It inhibits various strains of pathogenic avian influenza viruses in vitro and in vivo.
References
1) Ren?et al. (2011),?DNA hypermethylation as a chemotherapy target; Cell Signal,?23?1082 2) Gowher?et al. (2004),?Mechanism of inhibition of DNA methyltransferases by cytidine analogs in cancer therapy; Cancer Biol. Ther.,?3?1062 3) Zhao?et al. (2002),?Inhibition of cancer cell proliferation by 5-fluoro-2′-deoxycytidine, a DNA methylation inhibitor, through activation of DNA damage response pathway; Springerplus,?1?65 4) Kumaki?et al. (2001),?In vitro and in vivo efficacy of fluorodeoxycytidine analogs against highly pathogenic avian influenza H5N1, seasonal, and pandemic H1N1 virus infections; Antiviral Res.,?92?329
Properties of 2'-DEOXY-5-FLUOROCYTIDINE
| Melting point: | 196 °C |
| Boiling point: | 465℃ |
| Density | 1.82 |
| refractive index | 70 ° (C=1, H2O) |
| Flash point: | 235℃ |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: >20mg/mL |
| pka | 14.03±0.60(Predicted) |
| form | powder |
| color | white to off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 10356-76-0(CAS DataBase Reference) |
| EPA Substance Registry System | 5-Fluoro-2'-deoxycytidine (10356-76-0) |
Safety information for 2'-DEOXY-5-FLUOROCYTIDINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2'-DEOXY-5-FLUOROCYTIDINE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran





You may like
-
 2'-Deoxy-5-fluorocytidine CAS 10356-76-0View Details
2'-Deoxy-5-fluorocytidine CAS 10356-76-0View Details
10356-76-0 -
 5−Fluoro−2′−deoxycytidine CAS 10356-76-0View Details
5−Fluoro−2′−deoxycytidine CAS 10356-76-0View Details
10356-76-0 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0