2-Cyanopyridine
Synonym(s):2-Cyanopyridine;Picolinonitrile
- CAS NO.:100-70-9
- Empirical Formula: C6H4N2
- Molecular Weight: 104.11
- MDL number: MFCD00006218
- EINECS: 202-880-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-25 17:52:38
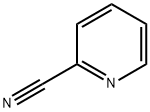
What is 2-Cyanopyridine?
Description
The cyanopyridines are as follows:2-cyano-: A white to tan liquid or solid with an almondodor. Boiling point=about 213℃; Freezing/Meltingpoint=27℃; Flash point=89℃.3-cyano-: A colorless liquid or gray crystalline solid.Molecular weight=104.12; Boiling point=83-84℃;Freezing/Melting point=47-49℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 3, Reactivity 0. Soluble in water.4-cyano-: A beige solid. Freezing/Melting point=75.8℃
Chemical properties
white to brown low melting crystalline solid
Chemical properties
The cyanopyridines are as follows: 2-cyano-: A white to tan liquid or solid. Almond odor. Boiling point=2213℃ ; freezing/melting point=27℃ ; flash point=89℃ . 3-cyano-: a colorless liquid or gray crystal- line solid.
The Uses of 2-Cyanopyridine
2-Cyanopyridine is a cyano substituted pyridine. 2-Cyanopyridine is a related compound of nicotine and is a component of tobacco smoke condensate.
The Uses of 2-Cyanopyridine
It is employed as an important chemical intermediate for rimiterol hydrobromide and used as a bronchodilator. It is also used as intermediates of pharmaceutical and dye and pigment. It acts as a precursor of the respective amidate for protein modification via amidation.
What are the applications of Application
2-Pyridinecarbonitrile is a precursor of the respective amidate for protein modification via amidation
Definition
ChEBI: A cyanopyridine carrying the cyano group at position 2.
Potential Exposure
Limits in Air NIOSH IDLH525 mg/m3 NIOSH REL: (nitriles) 2 ppm, Ceiling Concentration, not to be exceeded in any 15-minute work period.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from heat or flame and separate fromoxidizing materials
Shipping
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required, Potential Inhalation Hazard (Special Provision 5).
Incompatibilities
Oxidizing agents, such as perchlorates, peroxides, and permanganates. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give car- boxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .
Properties of 2-Cyanopyridine
| Melting point: | 24-27 °C(lit.) |
| Boiling point: | 212-215 °C(lit.) |
| Density | 1.081 g/mL at 25 °C(lit.) |
| vapor pressure | 11.96Pa at 25℃ |
| refractive index | n |
| Flash point: | 193 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 67g/l |
| form | Low Melting Crystalline Solid |
| pka | pK1:-0.26(+1) (25°C) |
| color | White to brown |
| PH | 8.4 (100g/l, H2O) |
| Water Solubility | immiscible |
| BRN | 107710 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Dielectric constant | 93.769999999999996 |
| CAS DataBase Reference | 100-70-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Pyridinecarbonitrile(100-70-9) |
| EPA Substance Registry System | 2-Pyridinecarbonitrile (100-70-9) |
Safety information for 2-Cyanopyridine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 2-Cyanopyridine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 100-70-9 98%View Details
100-70-9 98%View Details
100-70-9 -
 2-Cyanopyridine, ≥98% CAS 100-70-9View Details
2-Cyanopyridine, ≥98% CAS 100-70-9View Details
100-70-9 -
 2-Cyanopyridine CAS 100-70-9View Details
2-Cyanopyridine CAS 100-70-9View Details
100-70-9 -
 2-Pyridinecarbonitrile CAS 100-70-9View Details
2-Pyridinecarbonitrile CAS 100-70-9View Details
100-70-9 -
 2-CYANOPYRIDINE 100-70-9View Details
2-CYANOPYRIDINE 100-70-9View Details
100-70-9 -
 2 Cyano Pyridine, Grade Standard: Technical GradeView Details
2 Cyano Pyridine, Grade Standard: Technical GradeView Details
100-70-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
