2-Aminoethoxydiphenyl borate
Synonym(s):2-APB;2-Aminoethoxydiphenyl borate;2-Aminoethoxydiphenylborate, (2-Aminoethoxy)diphenylborane;2-Aminoethyl diphenyl borate;Diphenylboric acid 2-aminoethyl ester
- CAS NO.:524-95-8
- Empirical Formula: C14H16BNO
- Molecular Weight: 225.09
- MDL number: MFCD00014823
- EINECS: 208-366-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
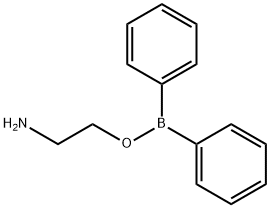
What is 2-Aminoethoxydiphenyl borate?
Description
2-Aminoethyl diphenylborinate (2-APB) is a modulator of intracellular calcium levels and transient receptor potential (TRP) channels. It inhibits calcium release induced by inositol-1,4,5-triphosphate (IP3) in rat cerebellar microsomes (IC50 = 42 μM) without affecting IP3 binding to its receptor. 2-APB inhibits store-operated calcium entry (SOCE) in DT40 cells (IC50 = 4.8 μM), as well as inhibits TRP canonical 6 (TRPC6) and TRP melastatin 8 (TRPM8) and activates TRP vanilloid 1 (TRPV1), TRPV2, and TRPV3 in vitro. It also inhibits calcium transients induced by the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin in DDT1 MF-2 cells when used at a concentration of 75 μM. 2-APB inhibits differentiation of primary mouse brown adipocytes when used at concentrations ranging from 30 to 300 μM and reduces adipocyte triglyceride levels at 100 μM. It also increases the expression of Ucp1 and Pparg in primary mouse brown adipocytes. 2-APB (10 mg/kg) reduces protein levels of TRP melastatin 2 (TRPM2) in the rat hippocampus and improves learning and memory deficits induced by amyloid-β (25-35) in rats.
Chemical properties
white crystalline powder
The Uses of 2-Aminoethoxydiphenyl borate
Ca release inhibitor, angiogensin II inhibitor
The Uses of 2-Aminoethoxydiphenyl borate
2-Aminoethyl diphenylborinate is used for the densitometric determination of flavonoids and TRP activator. It is also used to manipulate intracellular release of calcium ions and in preparation of apoptotic inducers. It serves as a reactant for preparation of apoptotic inducers and in membrane-permeable SOCE (store operated calcuym entry) inhibitor.
What are the applications of Application
2-APB is a TRP activator that also blocks IP3R
Definition
ChEBI: An organoboron compound that is diphenylborane in which the borane hydrogen is replaced by a 2-aminoethoxy group.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 5409, 1958 DOI: 10.1021/ja01553a022
Journal of the American Chemical Society, 80, p. 5409, 1958 DOI: 10.1021/ja01553a022
General Description
A cell-permeable modulator of Ins(1,4,5)P3-induced Ca2+ release. Inhibits Ins(1,4,5)P3-induced Ca2+ release from rat cerebellar microsomal preparations (IC50 = 42 μM) without affecting [3H]-Ins(1,4,5)P3 binding to its receptor. In liver cells, 2-APB inhibits store-operated Ca2+ channels through a mechanism which may involve its binding to either the channel protein or an associated regulatory protein. Has no effect on the Ca2+ release from the ryanodine-sensitive Ca2+ store prepared from rat leg skeletal muscle and heart.
Biological Activity
A functional and membrane permeable D-myo-inositol 1,4,5-trisphosphate (IP 3 ) receptor antagonist (IC 50 = 42 μ M). Stimulates store-operated calcium (SOC) release at low concentrations (< 10 μ M) and inhibits it at higher concentrations (up to 50 μ M). Increases STIM-Orai channel conductance and limits ion selectivity. Modulator of TRP channels; blocks TRPC1, TRPC3, TRPC5, TRPC6, TRPV6, TRPM3, TRPM7, TRPM8 and TRPP2 and at higher concentrations stimulates TRPV1, TRPV2 and TRPV3. Also blocks specific gap channel subtypes.
Biochem/physiol Actions
Membrane-permeable modulator of intracellular IP3-induced calcium release.
storage
Room temperature
References
1) Maruyama et al. (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release; J. Biochem., 122 498 2) Varnai et al. (2009) STIM and Orai: the long-awaited constituents of store-operated calcium entry; Trends Pharmacol. Sci., 30 118 3) Togashi et al. (2008) Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB); Br. J. Pharmacol., 153 1324 4) Xu et al. (2005) Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect; Br. J. Pharmacol., 145 405 5) Bai et al. (2006) Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB); J. Pharmacol. Exp. Ther., 319 1452
Properties of 2-Aminoethoxydiphenyl borate
| Melting point: | 192-194 °C(lit.) |
| Boiling point: | 325.3±34.0 °C(Predicted) |
| Density | 1.04±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in ethanol and methanol. |
| form | Liquid |
| pka | 8.03±0.10(Predicted) |
| color | Clear colorless to yellow |
| BRN | 2942273 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 524-95-8(CAS DataBase Reference) |
Safety information for 2-Aminoethoxydiphenyl borate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Aminoethoxydiphenyl borate
| InChIKey | BLZVCIGGICSWIG-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![Methanone, (3,4-dihydro-2(1H)-isoquinolinyl)[2-[(4-methoxyphenyl)amino]-4-thiazolyl]-](https://img.chemicalbook.in/CAS/20210111/GIF/736945-96-3.gif)
You may like
-
 2-Aminoethyl diphenylborinate, 97% CAS 524-95-8View Details
2-Aminoethyl diphenylborinate, 97% CAS 524-95-8View Details
524-95-8 -
 2-Aminoethyl Diphenylborinate CAS 524-95-8View Details
2-Aminoethyl Diphenylborinate CAS 524-95-8View Details
524-95-8 -
 2-APB CAS 524-95-8View Details
2-APB CAS 524-95-8View Details
524-95-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4