2-amino-5-methylbenzenesulfonamide
- CAS NO.:609-55-2
- Empirical Formula: C7H10N2O2S
- Molecular Weight: 186.23
- MDL number: MFCD00800465
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-18 09:04:46
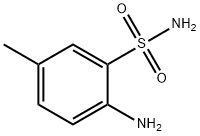
What is 2-amino-5-methylbenzenesulfonamide?
The Uses of 2-amino-5-methylbenzenesulfonamide
2-amino-5-methylbenzenesulfonamide is an important organic reagent that can be used as a building block for the synthesis of many organic compounds. It could reacted with triethyl orthoformate to give 7-Methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide by ring closure. It was an intermediate of potent AMPA receptor potentiators[1].
Synthesis
A solution of 7-methyl-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide (10, 10 g, 47,12 mmol) in sulfuric acid 50% (150 mL) was refluxed until complete dissolution of the suspension. At the end of the reaction, the solution was adjusted to pH 7 with an aqueous solution of concentrated NaOH. The solvent was removed by distillation under reduced pressure. The residue was triturated with methanol and the salts were eliminated by filtration. The filtrate was concentrated to dryness under reduced pressure and the residue was triturated in water (a minimum). The resulting precipitate was collected by filtration, washed with water, and dried to afford 2-amino-5-methylbenzenesulfonamide[1].
References
[1] Dintilhac G, et al. New substituted aryl esters and aryl amides of 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides as positive allosteric modulators of AMPA receptors. RSC Medicinal Chemistry, 2011; 2: 509-523.
Properties of 2-amino-5-methylbenzenesulfonamide
| storage temp. | 2-8°C |
| InChI | InChI=1S/C7H10N2O2S/c1-5-2-3-6(8)7(4-5)12(9,10)11/h2-4H,8H2,1H3,(H2,9,10,11) |
Safety information for 2-amino-5-methylbenzenesulfonamide
Computed Descriptors for 2-amino-5-methylbenzenesulfonamide
| InChIKey | GKTVIFGJASUBGA-UHFFFAOYSA-N |
| SMILES | C1(S(N)(=O)=O)=CC(C)=CC=C1N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1