2-AG
Synonym(s):(all-Z)-5,8,11,14-Eicosatetraenoic acid 2-hydroxy-1-(hydroxymethyl)ethyl ester;2-AG;2-arachidonoyl glycerol;2-Arachidonoylglycerol;2-Monoarachidonoylglycerol
- CAS NO.:53847-30-6
- Empirical Formula: C23H38O4
- Molecular Weight: 378.55
- MDL number: MFCD01862600
- EINECS: 200-835-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
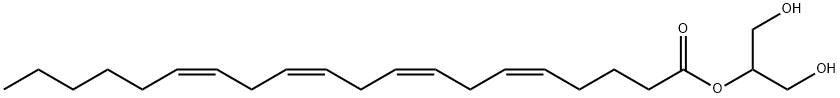
What is 2-AG?
The Uses of 2-AG
2-Arachidonoylglycerol is a major endocannabinoid, which can inhibit synaptic transmission by presynaptic cannabinoid CB1 receptors. It plays an inhibitory role in the bombesin-induced activation of central adrenomedullary outflow in rats.
Definition
ChEBI: An endocannabinoid and an endogenous agonist of the cannabinoid receptors (CB1 and CB2). It is an ester formed from omega-6-arachidonic acid and glycerol.
General Description
2-Arachidonoylglycerol (2-AG), is an endocannabinoid, an endogenous agonist of the CB1 receptor. 2-AG, unlike anandamide, is present at relatively high levels in the central nervous system; it is the most abundant molecular species of monoacylglycerol found in mouse and rat brain (~5-10 nmol/g tissue). Detection of 2-AG in brain tissue is complicated by the relative ease of its isomerization to 1-AG during standard lipid extraction conditions. Unlike anandamide, formation of 2-AG is calcium-dependent and is mediated by the activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL). 2-AG acts as a full agonist at the CB1 receptor. At a concentration of 0.3nM, 2-AG induces a rapid, transient increase in intracellular free calcium in NG108-15 neuroblastoma X glioma cells through a CB1 receptor-dependent mechanism. 2-AG is hydrolyzed in vitro by monoacylglycerol lipase (MAGL), fatty acid amide hydrolase (FAAH), and the uncharacterized serine hydrolase enzymes ABHD6 and ABHD12.
Biological Activity
Endogenous cannabinoid ligand that acts as a potent agonist at GPR55 (EC 50 values are 3, 519 and 618 nM at GPR55, CB 1 and CB 2 respectively; K i values are 472 and 1400 nM at CB 1 and CB 2 respectively). Found in the brain at concentrations 1000-fold higher than that of anandamide.
Biochem/physiol Actions
Endogenous cannabinoid receptor agonist.
Enzyme inhibitor
This potent endocannabinoid (FW = 378.30 g/mol; CAS 53847-30-6; Symbol: 2-AG), also known as 1,3-dihydroxy-2-propanyl (5Z,8Z,11Z,14Z)- 5,8,11,14-eicosatetraenoate, is an endogenous agonist of the CB1, the G- protein-coupled Cannabinoid receptor type-1 found primarily in the central and peripheral nervous system. 2-AG is found at highest concentrations in the CNS, where it exerts its cannabinoid-like neuromodulatory effects. Found in milk, 2-AG plays a role in sustaining infant suckling, and the selective CB1 receptor antagonist SR141617A (N-(piperidin-1-yl)-5-(4- chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3- carboxamide) permanently prevents milk ingestion in a dose-dependent manner, when administered to mouse pups, within 1 day of birth. 2-AG is formed by phospholipase C (PLC) and diacylglycerol lipase (DAGL) from arachidonic acid-containing diacylglycerol (DAG). In the CNS, three serine-hydrolases, monoacylglycerol lipase (MAGL), a,b-hydrolase- domain-6 (ABHD6) and a,b-hydrolase-domain 12 (ABHD12) are responsible for inactivation of the primary 2-arachidonoylglycerol. Irreversible ABHD6 inhibitors show exceptional potency and selectivity in cells (<5 nM) and, at equivalent doses in mice (1 mg/kg), acting as systemic and peripherally restricted inhibitors, respectively. Indeed, selective knockdown of ABHD6 in metabolic tissues protects mice from high-fat-diet-induced obesity, hepatic steatosis, and systemic insulin resistance.
storage
Store at -80°C
Properties of 2-AG
| Boiling point: | 508.6±50.0 °C(Predicted) |
| Density | 0.992±0.06 g/cm3(Predicted) |
| Flash point: | 2 °C |
| storage temp. | -20°C |
| solubility | DMSO: 10 mg/ml; Ethanol: Miscible; PBS (pH 7.2): ~150 μg/ml |
| pka | 13.54±0.10(Predicted) |
| form | acetonitrile solution |
| color | Colorless to light yellow |
| CAS DataBase Reference | 53847-30-6(CAS DataBase Reference) |
Safety information for 2-AG
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-AG
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![L-3-PHOSPHATIDYLCHOLINE,1-STEAROYL-2-[1-14C]ARACHIDONYL](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2470182.gif)
![1,2-PHOSPHATIDYLCHOLINE, L-ALPHA-DIARACHIDONYL, [DIARACHIDONYL-11-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB3140552.gif)
![PHOSPHATIDYLINOSITOL, L-ALPHA-1-STEAROYL-2- ARACHIDONYL, [ARACHIDONYL-1-14C]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9288405.gif)

![2-ARACHIDONYL GLYCEROL [ARACHIDONYL 5,6,8,9,11,12,14,15-3H(N)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB9359088.gif)
You may like
-
 2-Arachidonyl glycerol CAS 53847-30-6View Details
2-Arachidonyl glycerol CAS 53847-30-6View Details
53847-30-6 -
 2-AG CAS 53847-30-6View Details
2-AG CAS 53847-30-6View Details
53847-30-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8