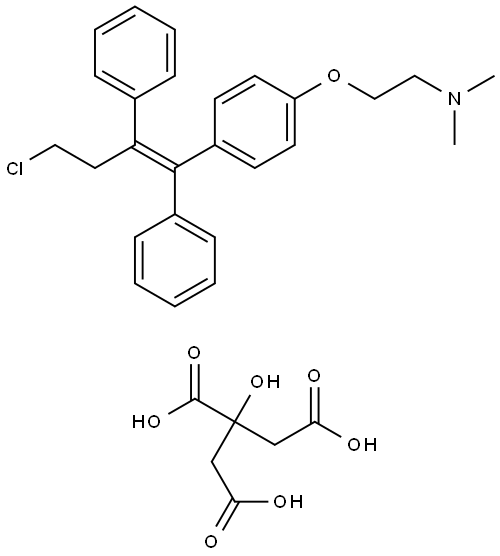
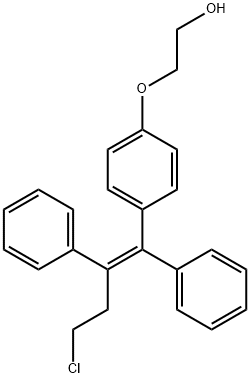
2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
Synonym(s):(deaminohydroxy)toremifene;2-[4-[(1Z)-4-Chloro-1,2-diphenyl-1-buten-1-yl]phenoxy]ethanol;FC-1271a
- CAS NO.:128607-22-7
- Empirical Formula: C24H23ClO2
- Molecular Weight: 378.89
- MDL number: MFCD00871890
- EINECS: 664-452-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-26 10:20:41
What is 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol?
Absorption
When a single oral dose of ospemifene 60 mg is given to postmenopausal women under fasted conditions, the pharmacokinetic parameters are as follows: Tmax = 2 hours (range of 1 - 8 hours); Cmax = 533 ng/mL; AUC (0-inf) = 4165 ng?hr/mL. When the same aforementioned dose is given to postmenopausal women under fed conditions, the pharmacokinetic parameters are as follows: Tmax = 2.5 hours (1 - 6 hours); Cmax = 1198 ng/mL; AUC (0-inf) = 7521 ng?hr/mL. Accumulation occurs following repeated doses. Time to steady state = 9 days. Although the bioavailability of ospemifene has not been formally evaluated, it is expected to have a low bioavailability because of its lipophilic nature.
Toxicity
Adverse reactions (≥1 percent) include: hot flush, vaginal discharge, muscle spasms, genital discharge, hyperhidrosis.
Description
In February 2013, the US FDA approved ospemifene (also referred to as FC1271a), for the treatment ofmoderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA), due tomenopause. It is estimated that there are 150 million postmenopausal women worldwide with 40–70% suffering from VVA. Ospemifene is a selective estrogen receptor (ER) modulator (SERM) and the first nonhormonal, nonestrogen for the treatment of moderate to severe dyspareunia in women with menopausal VVA. It binds to ERα (IC50~800 nM) and ERβ (IC50~1600 nM) with tissue-specific estrogenic agonist/antagonist effects. Treatment with ospemifene increases the thickness of the vaginal tissue thereby decreasing fragility of the tissue and reducing potential for pain during sexual intercourse.
Originator
Tess Diagnostics and Pharmaceuticals/Hormos Medical/QuatRx (Finland)
The Uses of 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
Treatment of vaginal atrophy, osteoporosis, and vasomotor symptoms.
Indications
Ospemifene is indicated for the treatment of moderate to severe dyspareunia and vaginal dryness associated with menopause.
Background
Ospemifene is a new selective non-hormonal estrogen receptor modulator (SERM) that is used for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. FDA approved on February 26, 2013.
Definition
ChEBI: An organochlorine compound that is a selective estrogen receptor modulator; used for treatment of dyspareunia.
brand name
Osphena
Pharmacokinetics
The half maximal inhibitory concentration (IC50) for estrogen receptor (ER) alpha and beta are 0.8 μM and 1.7 μM, respectively. Ospemifene has potential uses in the management of osteoporosis in postmenopausal women. It interacts with osteoblasts and osteoclasts in such a way that it reduces bone turnover. It also has potential uses in the prevention of breast cancer. Studies suggest that ospemifene, in a dose-dependent manner, reduces the incidence of tumours.
Clinical Use
Ospemifene is a SERM that is currently in Phase II/III clinical trials for the treatment of postmenopausal osteoporosis and urogenital atrophy. It is a known metabolite of toremifene, a triphenylethylene derivative used to treat breast cancer.Ospemifene has been shown to have beneficial effects on the bone without significant estrogen-related side effects. The beneficial effect observed on bone stems from this agent's ability to increase osteoblast proliferation and, as a result, to enhance bone mineralization as well as bone formation. Unlike tamoxifen, ospemifene does not induce osteocyte apoptosis.
Synthesis
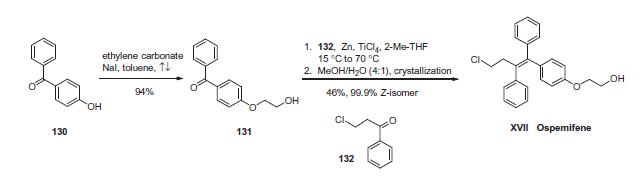
The drug can be synthesized succinctly in two steps. First, alkylation of commercially available 4-hydroxybenzophenone (130) with ethylene carbonate and catalytic sodium iodide in refluxing toluene provided benzophenone 131 in 94% yield. This was followed by a McMurry coupling involving benzophenone 131 with chloropropiophenone 132 in the presence of zinc powder and titanium tetrachloride in 2-methyltetrahydrofuran. This reaction gave rise to a mixture of triphenylethylenes directly as a 5.5:1 ratio of Z to E isomers which could be separated by crystallization in aqueous methanol to give a mixture of olefins, 98% of which was comprised of the desired Z-isomer corresponding to ospemifene (XVII). The product purity was further improved by recrystallization to give 99.9% of the Z-isomer in 46% yield from 131. Thus, ospemifene was synthesized in two steps and 43% overall yield.
Metabolism
Ospemifene is hepatically metabolized via CYP3A4, CYP2C9, CYP2C19, and CYP2B6. The major metabolite was 4-hydroxyospemifene, 25% of the parent compound will undergo this biotransformation. Other metabolites include 4'-hydroxy-ospemifene, <7% of the parent compound will undergo this biotransformation. In order of decreasing potency, ospemifene was suggested to be a weak inhibitor for CYP2B6, CYP2C9, CYP2C19, CYP2C8, CYP2D6 and CYP3A4.
Properties of 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
| Boiling point: | 544.6±50.0 °C(Predicted) |
| Density | 1.166±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 14.26±0.10(Predicted) |
| color | white to beige |
| CAS DataBase Reference | 128607-22-7 |
Safety information for 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
| InChIKey | LUMKNAVTFCDUIE-VHXPQNKSSA-N |
2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol manufacturer
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamine Radiator Flux Zinc Chloride Solution (All Grades) Levetiracetam Fmoc-Gln-OH Boc Phenyl Alanine Alpha Lipoic Acid*Related products of tetrahydrofuran
You may like
-
 128607-22-7 Ospemifene 98%View Details
128607-22-7 Ospemifene 98%View Details
128607-22-7 -
 128607-22-7 99%View Details
128607-22-7 99%View Details
128607-22-7 -
 Ospemifene 98%View Details
Ospemifene 98%View Details -
 128607-22-7 98%View Details
128607-22-7 98%View Details
128607-22-7 -
 Ospemifene CAS 128607-22-7View Details
Ospemifene CAS 128607-22-7View Details
128607-22-7 -
 Ospemifene CAS 128607-22-7View Details
Ospemifene CAS 128607-22-7View Details
128607-22-7 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0