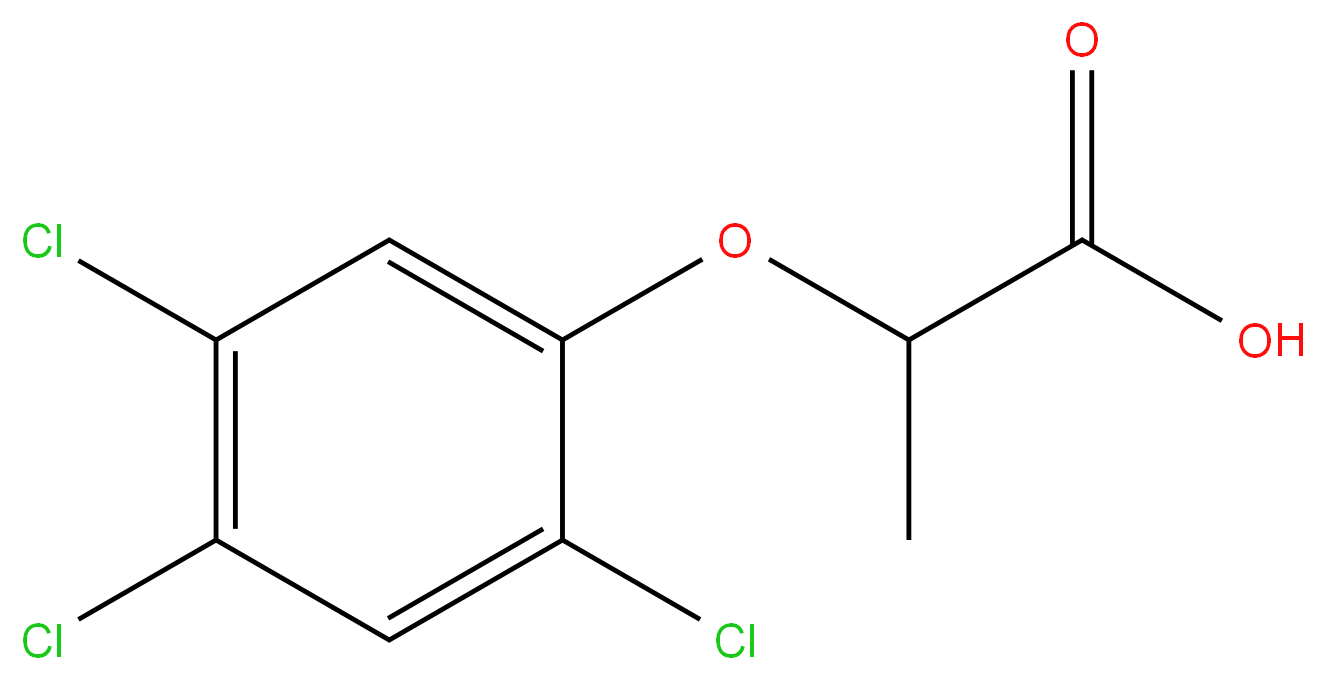
2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
Synonym(s):2-(2,4,5-Trichlorophenoxy)propionic acid;2-(2,4,5-Trichlorophenoxy)propionic acid solution;2,4,5-TP;Fenoprop;Silvex
- CAS NO.:93-72-1
- Empirical Formula: C9H7Cl3O3
- Molecular Weight: 269.51
- MDL number: MFCD00002646
- EINECS: 202-271-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
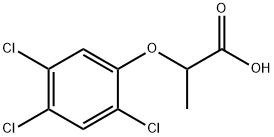
What is 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID?
Chemical properties
slightly beige crystalline powder
The Uses of 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
Herbicide and plant growth regulator.
The Uses of 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
Silvex was formerly used as a herbicide. Itsuse in rice fields, sugarcane, and orchards hasbeen stopped by the EPA.
Definition
ChEBI: Fenoprop is a monocarboxylic acid. It has a role as a phenoxy herbicide.
General Description
White powder. Sinks and mixes slowly with water.
Reactivity Profile
A halogenated organic acid derivative. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymer
Hazard
Use has been restricted.
Health Hazard
INHALATION: Irritating to nose and throat. May cause nausea, vomiting, lethargy and incoordination. May cause kidney and liver damage. EYES: Irritation. May cause corneal injury or burn. SKIN: Irritation.
Health Hazard
Silvex is moderately toxic to test animals.The toxic effects are comparable to thoseof 2,4-D and 2,4,5-T. Oral or subcutaneousadministration in mice caused embryo toxi-city and fetal death. The oral LD50 value inrats is 650 mg/kg.
Fire Hazard
Special Hazards of Combustion Products: Hydrogen chloride may be liberated.
Safety Profile
A suspected carcinogen. Poison by ingestion. An experimental teratogen. When heated to decomposition it emits toxic fumes of Cl-.
Properties of 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
| Melting point: | 175-180 °C |
| Boiling point: | 378.87°C (rough estimate) |
| Density | 1.5288 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 2.93±0.10(Predicted) |
| form | neat |
| Water Solubility | 71mg/L(25 ºC) |
| Merck | 13,8606 |
| BRN | 1985768 |
| Stability: | Stablr. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 93-72-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silvex (93-72-1) |
Safety information for 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H315:Skin corrosion/irritation H370:Specific target organ toxicity, single exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P321:Specific treatment (see … on this label). P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for 2-(2,4,5-TRICHLOROPHENOXY)PROPIONIC ACID
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamine Radiator Flux Zinc Chloride Solution (All Grades) Levetiracetam Fmoc-Gln-OH Boc Phenyl Alanine Alpha Lipoic Acid*Related products of tetrahydrofuran

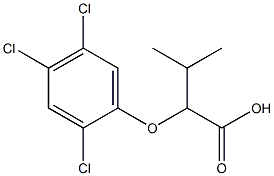

![[S,(-)]-2-(3,4,5-Trichlorophenoxy)propionic acid](https://img.chemicalbook.in/CAS/20200401/GIF/CB82261711.gif)
You may like
-
 93-72-1 Fenoprop 98%View Details
93-72-1 Fenoprop 98%View Details
93-72-1 -
 2-(2,4,5-Trichlorophenoxy)propionic acid CAS 93-72-1View Details
2-(2,4,5-Trichlorophenoxy)propionic acid CAS 93-72-1View Details
93-72-1 -
 2-(2,4,5-Trichlorophenoxy)propionic acid CAS 93-72-1View Details
2-(2,4,5-Trichlorophenoxy)propionic acid CAS 93-72-1View Details
93-72-1 -
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1
