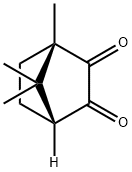
(1R)-(-)-CAMPHORQUINONE
Synonym(s):(1R)-(−)-2,3-Bornanedione;2,3-Bornanedione
- CAS NO.:10334-26-6
- Empirical Formula: C10H14O2
- Molecular Weight: 166.22
- MDL number: MFCD00082863
- EINECS: 626-988-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-31 13:32:20
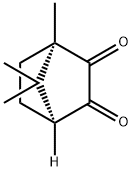
What is (1R)-(-)-CAMPHORQUINONE?
The Uses of (1R)-(-)-CAMPHORQUINONE
(1R)-(-)Camphorquinone are used as visible light photoinitiator for biomedical applications such as in dental surgery.
The Uses of (1R)-(-)-CAMPHORQUINONE
(1R)-(?)-Camphorquinone can be used as a chiral starting material for the preparation of:
- α-Hydroxycamphors by selective reduction of keto groups using various vegetables.
- Camphor-1,2-diamine platinum(II) complexes for DNA interaction studies.
- Camphoric anhydride by unsensitized photo-oxidation in the presence of oxygen and polar solvents.
- Camphorquinone-based chiral homoallylic amine, which is reacted with aldehydes to produce homoallylic primary amines via imine formation followed by 2-azonia-Cope rearrangement.
The Uses of (1R)-(-)-CAMPHORQUINONE
(1R)-(-)-Camphorquinone may be used as an analytical standard for the determination of the analyte in dental resin composite restorations in the oral environment in contact with food and beverages, and biological samples by analytical techniques.
What are the applications of Application
(1R)-(?)-Camphorquinone is A bioreductive, chiral starting material of quinones and other ketones by red algae.
Definition
ChEBI: (1R)-bornane-2,3-dione is a bornane-2,3-dione. It is an enantiomer of a (1S)-bornane-2,3-dione.
General Description
Camphorquinone, a 1,2-diketone, is a photoinitiator that finds wide use in the curing of resin composites. It functions by initiating the chain polymerization by free radical generation; typically with the aid of co-initiator amines.
Purification Methods
It can be purified by steam distillation, recrystallisation (yellow prisms) from EtOH, *C6H6 or Et2O/pet ether and it can be sublimed in a vacuum. The (±)-quinone forms needles from EtOH, m 197-198o, 203o. [Buxtorf & Flatt Helv Chim Acta 13 1026 1930, Asahena et al. Chem Ber 67 1432 1934, Beiltein 7 I 325.]
Properties of (1R)-(-)-CAMPHORQUINONE
| Melting point: | 200-203 °C(lit.) |
| Boiling point: | 254.44°C (rough estimate) |
| alpha | -101° (20/D)(c=2, C6H5CH3) |
| Density | 1.0060 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | almost transparency in Methanol |
| form | powder to crystal |
| color | Light yellow to Yellow |
| optical activity | [α]20/D 101°, c = 2 in toluene |
| BRN | 2327696 |
| CAS DataBase Reference | 10334-26-6(CAS DataBase Reference) |
Safety information for (1R)-(-)-CAMPHORQUINONE
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H334:Sensitisation, respiratory |
Computed Descriptors for (1R)-(-)-CAMPHORQUINONE
(1R)-(-)-CAMPHORQUINONE manufacturer
Opulant Pharmaceuticals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
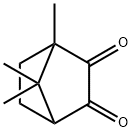
![7-(IODOMETHYL)-1,7-DIMETHYLBICYCLO[2.2.1]HEPTANE-2,3-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB8788478.gif)



You may like
-
 (1R)-(-)-Camphorquinone 98%View Details
(1R)-(-)-Camphorquinone 98%View Details -
 (1R)-(-)-Camphorquinone CAS 10334-26-6View Details
(1R)-(-)-Camphorquinone CAS 10334-26-6View Details
10334-26-6 -
 (1R)-(−)-Camphorquinone CAS 10334-26-6View Details
(1R)-(−)-Camphorquinone CAS 10334-26-6View Details
10334-26-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1