18-HYDROXY-11-DEOXYCORTICOSTERONE
- CAS NO.:379-68-0
- Empirical Formula: C21H30O4
- Molecular Weight: 346.46
- MDL number: MFCD00010486
- EINECS: 2068343
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-25 18:00:55
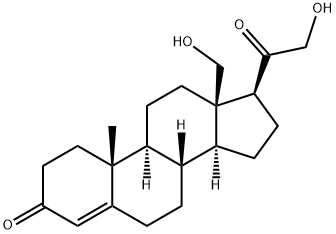
What is 18-HYDROXY-11-DEOXYCORTICOSTERONE?
Description
18-hydroxy-11-deoxy Corticosterone (18-OH-DOC) is a mineralocorticoid secreted by the zona fasciculata of the adrenal gland. Its biosynthesis is regulated by adrenocorticotropic hormone (ACTH; ) as well as angiotensin II , which increases 18-OH-DOC production in isolated human adrenal glomerulosa cells. 18-OH-DOC can be formed via conversion of 11-deoxy corticosterone (DOC; ) in human SK-MEL188 melanoma cells. 18-OH-DOC is an intermediate in the metabolism of progesterone and can be converted to aldosterone by the capsular portion of rat adrenal glands. Continuous infusion of 18-OH-DOC (200 μg/rat per day) increases systolic blood pressure in uninephrectomized saline-drinking rats. Plasma levels of 18-OH-DOC are elevated in a db/db mouse model of type 2 diabetes.
The Uses of 18-HYDROXY-11-DEOXYCORTICOSTERONE
11-Deoxy-18-hydroxycorticosterone is an analog of Corticosterone (C695700); a glucocorticoid and intermediate in the biosynthesis of Aldosterone (A514700) which is an adrenocortical steroid isolated from the adrenal cortex.
Definition
ChEBI: 18-hydroxydeoxycorticosterone is a 21-hydroxy steroid, a 3-oxo-Delta(4) steroid, a 20-oxo steroid and a 18-hydroxy steroid.
Purification Methods
Recrystallise 18-hydroxy-11-deoxycorticosterone from Et2O/Me2CO to give crystals m 200-205o. When it is recrystallised from M2CO, it has m 191-195o. It has UV with max at 240nm. The 21-O-acetoxy-18-hydroxy derivative has m 158-159o (from Et2O/*C6H6), and the 21-O-acetoxy-18,20-epoxy derivative has m 149-154o (from Et2O). [Kahnt et al. Helv Chim Acta 38 1237 1955; Pappo J Am Chem Soc 81 1010 1959.]
Properties of 18-HYDROXY-11-DEOXYCORTICOSTERONE
| Melting point: | 171-173 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly, Sonicated), Methanol (Slightly, Heated) |
| form | Solid |
| color | White to Off-White |
Safety information for 18-HYDROXY-11-DEOXYCORTICOSTERONE
Computed Descriptors for 18-HYDROXY-11-DEOXYCORTICOSTERONE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![ALDOSTERONE, D [1,2,6,7-3H(N)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5458996.gif)


![ALDOSTERONE, D-[1,2-3H(N)]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5241895.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1