15(S)-HETE
Synonym(s):(S)-15-HETE;(15S,5Z,8Z,11Z,13E)-15-Hydroxyeicosatetraenoic acid;15(S)-HETE
- CAS NO.:54845-95-3
- Empirical Formula: C20H32O3
- Molecular Weight: 320.47
- MDL number: MFCD00063590
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 20:41:25
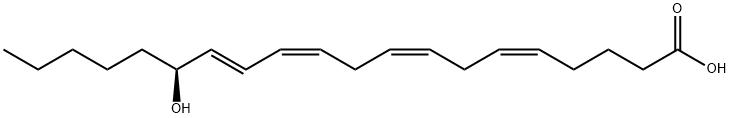
What is 15(S)-HETE?
Description
15(S)-
The Uses of 15(S)-HETE
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes. 15(S)-HETE is present in μg/ml concentrations in the nasal secretions of allergic rhinitis.
The Uses of 15(S)-HETE
15(S)-HETE is an arachidonic acid metabolite with immunosuppressive activity.
What are the applications of Application
15(S)-HETE is an arachidonic acid metabolite with immunosuppressive activity
Definition
ChEBI: An optically active form of 15-HETE having 15(S)-configuration..
General Description
15(S)-HETE is a product of the arachidonic acid pathway. It is synthesized by the human lungs and airway epithelial cells. 15(S)-HETE is a potent mucosecretagogue in human airways. It is a metabolite of 15-lipoxygenase. 15(S)-HETE is an endogenous ligand for peroxisome proliferator-activated receptor γ (PPARγ).
Biochem/physiol Actions
Metabolite of arachidonic acid via the 15-lipoxygenase pathway that may be involved in pulmonary anti-inflammatory responses through the inhibition of 5-lipoxygenase. 15(S)-HETE suppresses the incorporation of thymidine and biosynthesis of prostaglandin E2 in tumor cell cultures. Treatment of colon cancer cells with 15(S)-HETE inhibited cell proliferation and induced apoptosis, which was preceded by an increase in TGF-beta-inducible early gene (TIEG) and a decrease in Bcl-2.
Properties of 15(S)-HETE
| Boiling point: | 487.7±45.0 °C(Predicted) |
| Density | 0.984±0.06 g/cm3(Predicted) |
| Flash point: | 14℃ |
| storage temp. | -20°C |
| solubility | 0.1 M Na2CO3: 2 mg/ml; DMF: Miscible; DMSO: Miscible; Ethanol: Miscible; PBS (pH 7.2): 0.8 mg/ml |
| pka | 4.75±0.10(Predicted) |
| form | Liquid. |
| BRN | 2470466 |
Safety information for 15(S)-HETE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 15(S)-HETE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![15-HETE-[5,6,8,9,11,12,14,15-3H(N)]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB7158403.gif)
![15(S)-HETE [1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB2199997.gif)
![15(S)-HPETE [1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB6142441.gif)

![15(S)-HETE [3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB2439062.gif)
You may like
-
 15(S)-HETE CAS 54845-95-3View Details
15(S)-HETE CAS 54845-95-3View Details
54845-95-3 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4