1,5-Dihydroxy naphthalene
Synonym(s):1,5-Naphthalenediol
- CAS NO.:83-56-7
- Empirical Formula: C10H8O2
- Molecular Weight: 160.17
- MDL number: MFCD00003980
- EINECS: 201-487-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-22 13:59:06
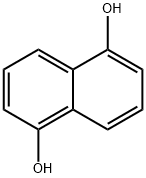
What is 1,5-Dihydroxy naphthalene?
Chemical properties
Grey powder 1,5-Naphthalenediol [83-56-7], 1,5-dihydroxynaphthalene, Azurol, C10H8O2, Mr 160.16, forms colorless needles (from aqueous methyl cellosolve). The compound is sublimable, readily soluble in ether and acetone, but only sparingly soluble in water and hydrocarbons. It is oxidized to 5-hydroxy-1,4-naphthoquinone ( juglone) by CrO3. Amination gives 1,5-naphthalenediamine; coupling with diazotized anilines occurs in the 2- or 4-position.
The Uses of 1,5-Dihydroxy naphthalene
1,5-Dihydroxynaphthalene is an intermediate of synthetic mordant azo dyes. It is an intermediate used in organic synthesis, pharmaceuticals, dyestuff fields and photograph industry.
Production Methods
The sodium salt of naphthalene-1,5-disulfonic acid is added gradually to excess 75 % sodium hydroxide. The mixture is heated under pressure to 276℃ over the course of 7 h and the reaction is completed by heating for a further 5 h at this temperature. After precipitation with sulfuric acid, the product is isolated in 90 % yield.
Purification Methods
The diol (~30g) is purified by making into a thick paste with H2O and suspending this in 3L of H2O containing 200mL of EtOH, boiling under reflux for 3hours, cooling to 30o, saturating with SO2, digesting below the boiling point for 1hour and filtering fast through a large hot filter paper. The hot filtrate is poured onto crushed ice whereby the diol (15-20g) separates as colourless needles (m 258o) [Wheeler & Ergle J Am Chem Soc 52 4873 1930]. Recrystallise it from H2O or nitromethane under N2 to avoid oxidation. The dibenzoyl derivative has m 245o (from EtOH). The 5-methoxy-1-naphthol derivative [prepared from the diol in MeOH/HCl (1:30 weight to volume ratio) and set aside at 25o for 9-10days] crystallised from pet ether (m 135-136o) or from CH2Cl2/hexane (needles m 140o) [Bell & McCaffrey Aust J Chem 46 731 1993]. [Beilstein 6 I 477, 6 II 950, 6 III 5265, 6 IV 6554.]
Properties of 1,5-Dihydroxy naphthalene
| Melting point: | 259-261 °C (dec.) (lit.) |
| Boiling point: | 246.06°C (rough estimate) |
| Density | 1.0924 (rough estimate) |
| vapor pressure | 0-0Pa at 25℃ |
| refractive index | 1.5418 (estimate) |
| Flash point: | 252°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 0.6g/l |
| form | Solid |
| pka | 9.28±0.40(Predicted) |
| color | Pale Beige to Very Dark Brown |
| PH | 6 (0.5g/l, H2O, 20℃) |
| Water Solubility | Soluble in water. |
| BRN | 2044951 |
| CAS DataBase Reference | 83-56-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,5-Naphthalenediol(83-56-7) |
| EPA Substance Registry System | 1,5-Naphthalenediol (83-56-7) |
Safety information for 1,5-Dihydroxy naphthalene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 1,5-Dihydroxy naphthalene
1,5-Dihydroxy naphthalene manufacturer
Prolife Bio Chemical Industries Pvt Ltd
LANXESS India Pvt. Ltd.
Santel Industries
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 1,5-dihydroxy naphthalene 99%View Details
1,5-dihydroxy naphthalene 99%View Details -
 1,5-dihydroxy naphthalene 98%View Details
1,5-dihydroxy naphthalene 98%View Details
83-56-7 -
 1,5-Dihydroxynaphthalene CAS 83-56-7View Details
1,5-Dihydroxynaphthalene CAS 83-56-7View Details
83-56-7 -
 1,5-Dihydroxynaphthalene, 97% CAS 83-56-7View Details
1,5-Dihydroxynaphthalene, 97% CAS 83-56-7View Details
83-56-7 -
 1,5-Dihydroxynaphthalene CAS 83-56-7View Details
1,5-Dihydroxynaphthalene CAS 83-56-7View Details
83-56-7 -
 1,5-dihydroxy naphthalene 83-56-7 98%View Details
1,5-dihydroxy naphthalene 83-56-7 98%View Details
83-56-7 -
 1,5-Dihydroxynaphthalene CAS 83-56-7View Details
1,5-Dihydroxynaphthalene CAS 83-56-7View Details
83-56-7 -
 1,5-Dihydroxynaphthalene CAS 83-56-7View Details
1,5-Dihydroxynaphthalene CAS 83-56-7View Details
83-56-7