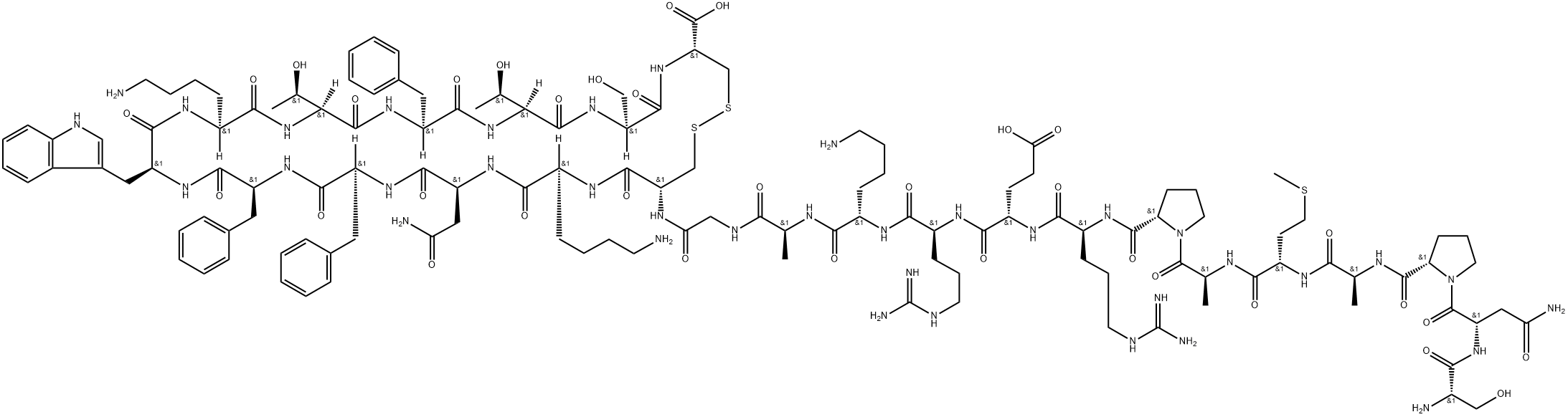
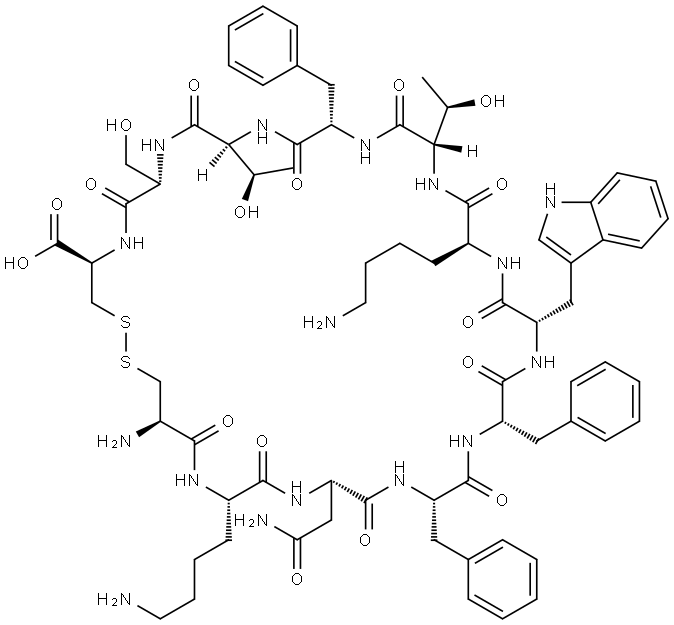
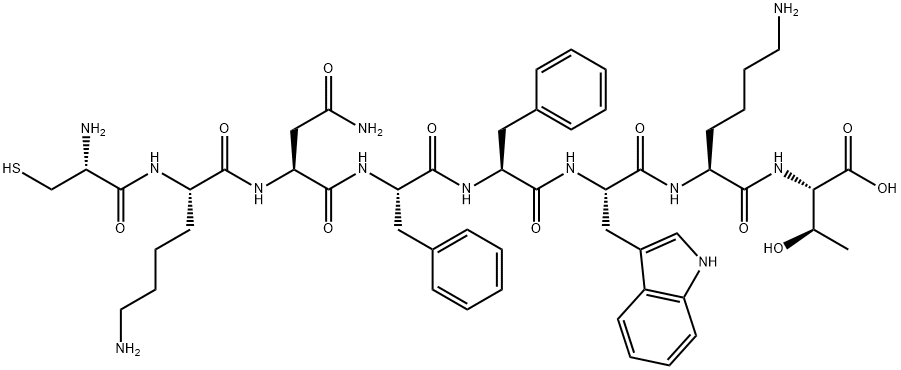
15-28-Somatostatin-28
- CAS NO.:38916-34-6
- Empirical Formula: C76H104N18O19S2
- Molecular Weight: 1637.88
- MDL number: MFCD00076762
- EINECS: 254-186-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33

What is 15-28-Somatostatin-28?
Absorption
This pharmacokinetic data is irrelevant.
Toxicity
Data is not available.
Chemical properties
White Powder
The Uses of 15-28-Somatostatin-28
Growth hormone-release inhibiting factor; treatment of severe, acute hemorrhage of gastroduodenal ulcers; experimental antidiabetic
The Uses of 15-28-Somatostatin-28
Growth hormone-release inhibiting factor; treatment of severe, acute hemorrhage of gastroduodenal ulcers; experimental antidiabetic.
Background
Somatostatin, also known as growth hormone-inhibiting hormone, is a naturally-occurring peptide hormone of 14 or 28 amino acid residues that regulates the endocrine system. It is secreted by the D cells of the islets to inhibit the release of insulin and glucagon, and is also generated in the hypothalamus, where it inhibits the release of growth hormone and thyroid-stimulating hormones from the anterior pituitary . Somatostatin is initially secreted as a 116 amino acid precursor, preprosomatostatin, which undergoes endoproteolytic cleavage to prosomastatin. Prosomastatin is further process into two active forms, somatostatin-14 (SST-14) and somatostatin-28 (SST-28), an extended SST-14 sequence to the N-terminus . The actions of somatostatin are mediated via signalling pathways of G protein-coupled somatostatin receptors.
Antineoplastic effects and potential uses of somatostatin on various tumours, including pituitary adenomas, GEP-NETs, paragangliomas, carcinoids, breast cancers, malignant lymphoma and small-cell lung cancers, have been extensively investigated . Somatostatin has been used in the clinical setting for the diagnosis of acromegaly and gastrointestinal tract tumours. Its analogues have been developed to achieve more favourable kinetics for efficiency use in the management of acute conditions, such as esophageal varices. Octreotide is a long-acting analogue of somatostatin that inhibits the release of a number of hormones, and is clinically used to relieve symptoms of uncommon gastroenteropancreatic endocrine tumours, as well as treat acromegaly .
Indications
For the symptomatic treatment of acute bleeding from esophageal varices. Other treatment options for long-term management of the condition may be considered if necessary, once initial control has been established.
Definition
ChEBI: A fourteen-membered heterodetic cyclic peptide comprising the sequence Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys cyclised by a disulfide bridge between the two Cys residues at positions 3 and 14.
Biological Functions
Somatostatin is a cyclic 14-peptide that was first isolated by Guillemin in 1973 and is probably the most thoroughly investigated and most important of the inhibitory factors produced by the hypothalamus. The principal activity of somatostatin, which is of hypothalamic origin, is inhibition of the release of growth hormone (GH) from the anterior pituitary. Too much GH, as in pituitary tumors, causes acromegaly, a form of giantism. On the other hand, too little GH leads to dwarfism. Somatostatin also has been found in the pancreas and the GI tract, where it inhibits the secretion of both insulin and glucagon from the pancreas as well as the secretion of a variety of intestinal peptides (e.g., gastrin, secretin, pepsin, and renin). The short half-life of somatostatin, which is less than 3 minutes, has precluded its use as a therapeutic agent. Many derivatives of somatostatin have been prepared to increase its duration of action and/or to enhance its selectivity of action. The culmination of these structure–activity studies was the development of octreotide acetate.
General Description
Somatostatin (SST) is a peptide.
Biochem/physiol Actions
Somatostatin regulates the secretion of hormones and bioactive peptides. It acts as an inhibitor in the digestive tract by inhibiting gastrin release. In the pancreatic islets, it inhibits glucagon and insulin release. Somatostatin and its synthetic analogs have been used in the treatment of neuroendocrine tumors.
Pharmacokinetics
Somatostatin is an endogenous peptide hormone that is secreted by the central nervous system, gastrointestinal tract, retina, peripheral neurons and pancreatic D cells of the islets of Langerhans. It exhibits several biological roles but predominantly exerts an inhibitory effect on secretion of other hormones and transmitters . While distribution of two active isoforms of somatostatin is similar, SST-14 is more predominant in the enteric neurons and peripheral nerves whereas SST-28 is more prominent in the retina and intestinal mucosal cells .
Anterior pituitary gland and brain: It inhibits the release of growth hormones and thyroid-stimulating hormones, such as thyroid stimulating hormone (TSH) and thyrotrophin, from the anterior pituitary while inhibiting the release of dopamine from the midbrain, norepinephrine, TRH and corticotrophin-releasing hormone in the brain .
Pancreas: In the pancreas, somatostatin reduces the secretion of glucagon and insulin as well as bicarbonate ions and other enzymes .
Thyroid gland: Somatosatin reduces secretion of T3, T4, and calcitonin . Somatostatin regulates the thyroid function by reducing basal TSH release .
Gastrointestinal tract: It attenuates the release of most gastrointestinal hormones such as gastrin, secretin, motilin, gastric acid, enteroglucagon, cholecystokinin (CCK), vasoactive intestinal peptide (VIP), gastric inhibitory polypeptide (GIP), intrinsic factor, pepsin, neurotensin, as well as bile secretion and colonic fluid secretion .
Adrenal gland: It inhibits angiotensin II-stimulated aldosterone secretion and acetylcholine-induced medullary catecholamine secretion .
Eye/retina: Somatostatin inhibits the production of vascular endothelial growth factor .
Inflammatory cells and sensory nerves: The expression of somatostatin has been found in inflammatory cells such as lymphocytes, monocytes, macrophages and endothelial cells to act as an autocrine or paracrine regulator in local immune responses. Findings suggest that somatostatin may play a role in exerting local and systemic anti-inflammatory and antinociceptive effects . On primary afferent neurons, somatostatin reduces the responses to thermal stimulation in C-mechanoheat sensitive fibers in a dose-dependent fashion and reduces the responses of C-mechanoheat fibers to bradykinin-induced excitation and sensitization to heat . Somatostatin is reported to elicit an analgesic effect when applied intrathecally; there is evidence supporting that similar effects may occur when systemically used to treat endocrine disorders .
Somatostatin is thought to reduce bleeding from esophageal varices by causing splanchnic vasoconstriction . Somatostatin elicits anti-neoplastic actions on various tumours via direct or indirect effects, or a combination of both .
Metabolism
Somatostatin is rapidly degraded by peptidase enzymes present in cells and plasma .
Purification Methods
Somatostatin is a tetradecapeptide which is purified by gel filtration on Sephadex G-25, eluting with 2N AcOH, and then by liquid partition chromatography on Sephadex G-25 using n-BuOH/AcOH/H2O (4:1:5) and has RF = 0.4. It is a brain growth hormone releasing-inhibiting factor which has also been synthesised. [Burgus et al. Proc Natl Acad Sci USA 70 684 1973, Sorantakis & McKinley Biochem Biophys Res Commun 54 234 1973, Hartridt et al. Pharmazie 37 403 1982.]
Properties of 15-28-Somatostatin-28
| Melting point: | >211°C (dec.) |
| Boiling point: | 1970.9±65.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | H2O: 1 mg/mL |
| form | powder |
| pka | 2.94±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in water(25,00°C 1,00 g/L), Methanol. |
| Merck | 13,8788 |
| BRN | 6436064 |
Safety information for 15-28-Somatostatin-28
Computed Descriptors for 15-28-Somatostatin-28
| InChIKey | NHXLMOGPVYXJNR-ATOGVRKGSA-N |
15-28-Somatostatin-28 manufacturer
Ralington Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 38916-34-6 Somatostatin 98%View Details
38916-34-6 Somatostatin 98%View Details
38916-34-6 -
 Somatostatin CAS 38916-34-6View Details
Somatostatin CAS 38916-34-6View Details
38916-34-6 -
 Somatostatin CAS 38916-34-6View Details
Somatostatin CAS 38916-34-6View Details
38916-34-6 -
 Somatostatin CAS 38916-34-6View Details
Somatostatin CAS 38916-34-6View Details
38916-34-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1