1,3,5,7-Cyclooctatetraene
Synonym(s):[8]Annulene;Cancer Osaka thyroid oncogene;C-COT;COT proto-oncogene serine/threonine-protein kinase;TPL-2
- CAS NO.:629-20-9
- Empirical Formula: C8H8
- Molecular Weight: 104.15
- MDL number: MFCD00004161
- EINECS: 211-080-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-26 11:30:52
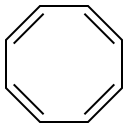
What is 1,3,5,7-Cyclooctatetraene?
Chemical properties
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
Physical properties
Cyclooctatetraene (COT) is a poster child for nonaromatic molecules. An isomer of tub-shaped COT, with one of the ring double bonds changed from the usual cis form to a trans one, lies some 23 kcal/mol higher in energy.
Cyclooctatetraene is an 8p electron system and has a triplet ground state if the p electrons are delocalized. It is antiaromatic hence highly unstable and reactive. It adopts a non-planar boat conformation to attain stability with alternating single and double bonds and hence behaves like a polyolefin.
Cyclooctatetraene is a nonaromatic 4n π-conjugated system with a non-planar tub-shaped geometry and potential energy surfaces at the ground state.
The Uses of 1,3,5,7-Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is used in the synthesis of highly organic film for silicon surfaces to improve its chemical and physical properties. It is also used in liquid state organic dye lasers, as triplet state quencher to reduce dye blinking.
The Uses of 1,3,5,7-Cyclooctatetraene
Used in the synthesis of highly organic film for silicon surfaces to improve its chemical and physical properties. 1 Used in liquid state organic dye lasers. 2 Used as triplet state quencher to reduce dye blinking. 3
The Uses of 1,3,5,7-Cyclooctatetraene
COT may be functionalized by two side groups which can be used as active anode materials for rechargeable batteries. High capacity and voltage organic cathodes may be developed by using COT that can be fused with carbon molecules.
What are the applications of Application
Cyclooctatetraene is an anti-aromatic hydrocarbon with polyolefin-like behavior
Definition
ChEBI: An antiaromatic annulene that is cyclooctane having four double bonds at positions 1, 3, 5 and 7.
Synthesis Reference(s)
Tetrahedron Letters, 21, p. 4791, 1980 DOI: 10.1016/0040-4039(80)80141-9
General Description
A colorless liquid. May irritate skin and eyes. Less dense than water and insoluble in water. Flash point 70°F. Vapors heavier than air. Used to make rubber.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1,3,5,7-CYCLOOCTATETRAENE may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Purification Methods
Purify the triene by shaking 3mL with 20mL of 10% aqueous AgNO3 for 15minutes, then filtering off the AgNO3 complex which precipitates. The precipitate is dissolved in water and added to cold concentrated ammonia to regenerate the cyclooctatetraene which is fractionally distilled under vacuum onto molecular sieves and stored at 0o. It is passed through a dry alumina column before use [Broadley et al. J Chem Soc, Dalton Trans 373 1986]. [Beilstein 5 I 228, 5 IV 1331.]
Properties of 1,3,5,7-Cyclooctatetraene
| Melting point: | -5--3 °C (lit.) |
| Boiling point: | 142-143 °C (lit.) |
| Density | 0.925 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 72 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Soluble), Methanol (Soluble) |
| form | Liquid |
| Specific Gravity | 0.943 |
| color | Turbid yellow to yellow-brown |
| Water Solubility | Not miscible or difficult to mix in water. |
| Sensitive | Light Sensitive |
| BRN | 2496800 |
| Dielectric constant | 2.46 |
| Stability: | Stability Unstable - commercial product may be stabilized by the addition of a small amount of hydroquinone. May form explosive peroxides in storage. Do not distill to small volume. Readily forms explosive mixtures with air. Flammable. Incompatible with air, strong oxidizing agents. |
| CAS DataBase Reference | 629-20-9(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3,5,7-Cyclooctatetraene (629-20-9) |
Safety information for 1,3,5,7-Cyclooctatetraene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,3,5,7-Cyclooctatetraene
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1,3,5,7-Cyclooctatetraene (stabilized with HQ) CAS 629-20-9View Details
1,3,5,7-Cyclooctatetraene (stabilized with HQ) CAS 629-20-9View Details
629-20-9 -
 Cyclooctatetraene CAS 629-20-9View Details
Cyclooctatetraene CAS 629-20-9View Details
629-20-9 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4