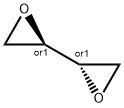
1,3-Butadiene diepoxide
Synonym(s):1,2,3,4-Diepoxybutane
- CAS NO.:1464-53-5
- Empirical Formula: C4H6O2
- Molecular Weight: 86.09
- MDL number: MFCD00005120
- EINECS: 215-979-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
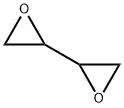
What is 1,3-Butadiene diepoxide?
Description
Diepoxybutane is a flammable, colorless liquid. Molecular weight=86.09; Boiling point=57℃ at25℃; Freezing/Melting point=19℃; also reported at2-4℃; Flash point=44.8℃. Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 0,Reactivity 0. Mixes with water.
Chemical properties
clear colorless to very slightly yellow liquid
The Uses of 1,3-Butadiene diepoxide
1,3-Butadiene Diepoxide is used as a substance in the evaluation of anti-diabetic and anti-ulcer potential of selected fresh fruits samples and their GC/MS analysis. Also in the inhibitory potency of 4-carbon alkanes and alkenes towards CYD2E1 activity.
The Uses of 1,3-Butadiene diepoxide
Diepoxybutane has been used as a research chemical, as a chemical intermediate, as a curing agent for polymers, as a cross-linking agent for textile fabrics, and to prevent microbial spoilage (IARC 1976, HSDB 2009). It has also been used to synthesize erythritol and pharmaceuticals (HSDB 2009). Because of its cross-linking properties, diepoxybutane is used as a test agent in the diagnosis of Fanconi anemia, which is characterized by chromosomal instability (Auerbach et al. 1989).
The Uses of 1,3-Butadiene diepoxide
Curing of polymers; cross-linking of textile fibers; prevention of microbial spoilage
The Uses of 1,3-Butadiene diepoxide
Butadiene diepoxide is used as a research chemical, as a chemical intermediate, as a curing agent for polymers, as a cross-linking agent for textile fabrics, and to prevent microbial spoilage. It is also been used to synthesize erythritol and pharmaceuticals.
Production Methods
Diepoxybutane is prepared by chlorination of butadiene followed by epoxidation with peracetic acid, with subsequent hydrolysis of the epoxide group and final reepoxidation with caustic.
Definition
ChEBI: Diepoxybutane is an epoxide. It has a role as a mutagen.
General Description
Colorless liquid. Used in curing polymers; crosslinking textile fibers and to prevent spoilage.
Air & Water Reactions
Highly Flammable
Reactivity Profile
Epoxides, such as 1,3-BUTADIENE DIEPOXIDE, are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Hazard
Probable carcinogen; neoplastigen; tumorigenic; poison; mutagen.
Health Hazard
Severe skin and eye irritant. Accidental minor exposure caused swelling of the eyelids, upper respiratory tract irritation and painful eye irritation 6 hours after exposure.NOTE: The dl- and meso-forms (CAS Registry Numbers 298-18-0 and 564-00-1, respectively) as well as the L(-) form (CAS Registry Number 30031-64-2) have all been determined to be positive animal carcinogens.
Fire Hazard
When heated to decomposition 1,3-BUTADIENE DIEPOXIDE emits acrid smoke and irritating fumes.
Safety Profile
Confirmed carcinogen withexperimental tumorigenic data. Poison by ingestion,inhalation, skin contact, and intraperitoneal routes.Human mutation data reported. A severe skin and eyeirritant. When heated to decomposition it emits acridsmoke and irritat
Potential Exposure
DEB is primarily used in researchand experimental work; as a curing agent for polymers; asa crosskicking agent for textile fabrics; and in preventingmicrobial spoilage in substances. DEB is also used commercially as mixed stereoisomers and as individual isomers in the preparation of erythritol and otherpharmaceuticals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Diepoxybutane is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with diepoxybutane you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
Toxic liquids, flammable, organic, n.o.s. requirea shipping label of “POISONOUS/TOXIC MATERIALS,FLAMMABLE LIQUID.” It falls in Hazard Class 6.1 andPacking Group I.
Properties of 1,3-Butadiene diepoxide
| Melting point: | 2-4 °C(lit.) |
| Boiling point: | 56-58 °C25 mm Hg(lit.) |
| alpha | 0°(neat) |
| Density | 1.113 g/mL at 25 °C(lit.) |
| vapor pressure | 25 mm Hg ( 56 °C) |
| refractive index | n |
| Flash point: | 114 °F |
| storage temp. | 0-6°C |
| form | neat |
| color | Colorless liquid |
| Specific Gravity | 1.1 |
| Water Solubility | Soluble in water (1000 g/L). |
| Merck | 14,3676 |
| BRN | 79831 |
| CAS DataBase Reference | 1464-53-5(CAS DataBase Reference) |
| IARC | (Vol. 11, Sup 7) 1987 |
| EPA Substance Registry System | Diepoxybutane (1464-53-5) |
Safety information for 1,3-Butadiene diepoxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H301:Acute toxicity,oral H314:Skin corrosion/irritation H340:Germ cell mutagenicity H350:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 1,3-Butadiene diepoxide
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 1,3-Butadiene diepoxide CAS 1464-53-5View Details
1,3-Butadiene diepoxide CAS 1464-53-5View Details
1464-53-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
