1,3-Acetonedicarboxylic acid
Synonym(s):ß-Ketoglutaric acid, Acetone-1,3-dicarboxylic acid;3-Oxoglutaric acid
- CAS NO.:542-05-2
- Empirical Formula: C5H6O5
- Molecular Weight: 146.1
- MDL number: MFCD00002711
- EINECS: 208-797-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
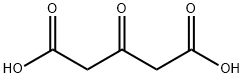
What is 1,3-Acetonedicarboxylic acid?
Chemical properties
white to off-white powder
Physical properties
Acetonedicarboxylic acid forms colorless crystals that are readily soluble in water and ethanol, and sparingly soluble in trichloromethane and diethyl ether. It melts with decomposition at 138 ℃.
The Uses of 1,3-Acetonedicarboxylic acid
1,3-Acetonedicarboxylic acid has been applied as a stimuli for reversible time-controlled pH modulation. It is cheaper and safer than commonly used reagents and enables the switch of the pH of a chemical system through its catalyzed decarboxylation. This was notably applied in the reversible disruption of a hydrogel. It is used as a building block in organic chemistry. It is also used in synthesising hetrocyclic rings and in the Weiss-Cook reaction, which involves the preparation of cis-bicyclo[3.3.0]octane-3,7-dione by reacting with diacyl (1,2 ketone). Its presence in human urine is used as a diagnostic test for the overgrowth of harmful gut flora, such as Candida albicans. 1,3-Acetonedicarboxylic acid is used in the synthesis of benzodiazepine derivatives. Also used in the synthesis of orally available hepatitis C virus polymerase inhibitors.
Definition
ChEBI: 3-Oxoglutaric acid is an oxo carboxylic acid.
Production Methods
1,3-Acetonedicarboxylic acid is produced from citric acid [77-92-9] by many industrial processes that differ only slightly. Citric acid is treated with oleum and reacts to yield acetonedicarboxylic acid via decarbonylation and dehydration.
Other possible production methods include reaction of acetone with carbon dioxide, oxidation of citric acid with chlorosulfuric acid, or reaction of ketene with phosgene.
Flammability and Explosibility
Non flammable
Description
1,3-Acetonedicarboxylic acid is an intriguing molecule possessing two pendant carboxylic acid functions that can potentially be involved in double biomimetic-like decarboxylative enolate formation, rendering the corresponding acetone α,α′-dianion readily available. Prepared on a large scale from raw material, namely citric acid, it can act as a potentially reactive di-nucleophilic acetone surrogate. Robinson famously applied this property in his 1917 tropinone synthesis and has since then found intensive utilization in the synthesis of tropinone-like skeletons.
Purification Methods
Crystallise it from ethyl acetate and store it over P2O5. It decarboxylates in hot water. [Beilstein 3 IV 1816.]
Properties of 1,3-Acetonedicarboxylic acid
| Melting point: | 133 °C (dec.) (lit.) |
| Boiling point: | 185.67°C (rough estimate) |
| Density | 1.2821 (rough estimate) |
| vapor pressure | 0.005Pa at 25℃ |
| refractive index | 1.3920 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | pK (25°) 3.10 |
| form | Crystalline Powder |
| color | White |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,68 |
| BRN | 1447081 |
| Stability: | Unstable in Solution |
| CAS DataBase Reference | 542-05-2(CAS DataBase Reference) |
| EPA Substance Registry System | Pentanedioic acid, 3-oxo- (542-05-2) |
Safety information for 1,3-Acetonedicarboxylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 1,3-Acetonedicarboxylic acid
| InChIKey | OXTNCQMOKLOUAM-UHFFFAOYSA-N |
1,3-Acetonedicarboxylic acid manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 542-05-2 1,3-Acetonedicarboxylic acid 98%View Details
542-05-2 1,3-Acetonedicarboxylic acid 98%View Details
542-05-2 -
 1,3-Acetonedicarboxylic Acid CAS 542-05-2View Details
1,3-Acetonedicarboxylic Acid CAS 542-05-2View Details
542-05-2 -
 3-Oxoglutaric acid CAS 542-05-2View Details
3-Oxoglutaric acid CAS 542-05-2View Details
542-05-2 -
 1,3-Acetonedicarboxylic acid CAS 542-05-2View Details
1,3-Acetonedicarboxylic acid CAS 542-05-2View Details
542-05-2 -
 Industrial Grade 1,3-Acetonedicarboxylic Acid CAS NO. 542-05-2View Details
Industrial Grade 1,3-Acetonedicarboxylic Acid CAS NO. 542-05-2View Details
542-05-2 -
 3- Oxoglutaric Acid, Packaging Type: Bag, Packaging Size: 25 kgView Details
3- Oxoglutaric Acid, Packaging Type: Bag, Packaging Size: 25 kgView Details
542-05-2 -
 Transparent Industrial Grade 3-Oxoglutaric Acid Cas: 542-05-2, 99%View Details
Transparent Industrial Grade 3-Oxoglutaric Acid Cas: 542-05-2, 99%View Details
542-05-2 -
 3-oxopentanedioic acid 542-05-2 99.0%View Details
3-oxopentanedioic acid 542-05-2 99.0%View Details
542-05-2
