1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
- CAS NO.:321-64-2
- Empirical Formula: C13H14N2
- Molecular Weight: 198.26
- MDL number: MFCD00046923
- EINECS: 206-291-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
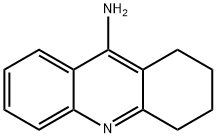
What is 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE?
Absorption
Tacrine is rapidly absorbed. Absolute bioavailability of tacrine is approximately 17%.
Toxicity
Overdosage with cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. The estimated median lethal dose of tacrine following a single oral dose in rats is 40 mg/kg, or approximately 12 times the maximum recommended human dose of 160 mg/day.
Description
In the 1950s, tacrine was used experimentally to reverse cholinergic coma in animals. In the 1960s, tacrine was used to reverse the effects of phencyclidine-like drugs. It was also marketed for many years as a respiratory stimulant. In 1993, the US Food and Drug Administration approved tacrine for the treatment of symptoms of mild to moderate Alzheimer’s disease.
The Uses of 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
The current use of tacrine is limited due to its poor oral bioavailability, the necessity for four daily doses, and serious side effects (including nausea, vomiting, dry mouth, indigestion, diarrhea, loss of appetite, urinary incontinence, collapse, convulsions, and hepatotoxicity). Currently, newer cholinesterase inhibitors (such as donepezil, rivastigmine, and galantamine) are preferred over tacrine.
Indications
For the palliative treatment of mild to moderate dementia of the Alzheimer's type.
Background
A centerally active cholinesterase inhibitor that has been used to counter the effects of muscle relaxants, as a respiratory stimulant, and in the treatment of Alzheimer's disease and other central nervous system disorders. Tacrine has been discontinued for the United States market.
Definition
ChEBI: Tacrine is a member of the class of acridines that is 1,2,3,4-tetrahydroacridine substituted by an amino group at position 9. It is used in the treatment of Alzheimer's disease. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor. It is a member of acridines and an aromatic amine. It is a conjugate base of a tacrine(1+).
Synthesis Reference(s)
Tetrahedron Letters, 4, p. 1277, 1963 DOI: 10.1039/jr9630005127
Pharmacokinetics
Tacrine is a parasympathomimetic- a reversible cholinesterase inhibitor that is indicated for the treatment of mild to moderate dementia of the Alzheimer's type. An early pathophysiological feature of Alzheimer's disease that is associated with memory loss and cognitive deficits is a deficiency of acetylcholine as a result of selective loss of cholinergic neurons in the cerebral cortex, nucleus basalis, and hippocampus. Tacrine is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine at cholinergic synapses through reversible inhibition of its hydrolysis by acetylcholinesterase. If this proposed mechanism of action is correct, tacrine's effect may lessen as the disease progresses and fewer cholinergic neurons remain functionally intact. There is no evidence that tacrine alters the course of the underlying dementing process.
Metabolism
Hepatic. Cytochrome P450 1A2 is the principal isozyme involved in tacrine metabolism. The major metabolite, 1-hydroxy-tacrine (velnacrine), has central cholinergic activity.
Toxicity evaluation
Tacrine has numerous mechanisms of action. The putative
principal mechanism of action of tacrine for Alzheimer’s
disease is reversible inhibition of acetylcholinesterase (AChE),
which thereby slows the breakdown of the chemical messenger
acetylcholine (ACh) in the brain. Tacrine also inhibits butyrylcholinesterase
activity. In addition, tacrine blocks sodium
and potassium channels. Tacrine also acts as a histamine
N-methyltransferase inhibitor.
At a therapeutic dose, tacrine causes liver toxicity. Cytotoxicity
studies using the human liver cell line HepG2 showed that
a therapeutic blood concentration of tacrine induces reactive
oxygen species production and glutathione depletion, suggesting
that oxidative stress might be involved in tacrine
hepatotoxicity.
Properties of 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
| Melting point: | 183.5℃ |
| Boiling point: | 325.59°C (rough estimate) |
| Density | 0.9827 (rough estimate) |
| refractive index | 1.4400 (estimate) |
| storage temp. | 4°C, away from moisture and light |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 9?+-.0.20(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| CAS DataBase Reference | 321-64-2(CAS DataBase Reference) |
Safety information for 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4