1,1,3,3-Tetramethylurea
Synonym(s):1,1,3,3-Tetramethylurea;TMU
- CAS NO.:632-22-4
- Empirical Formula: C5H12N2O
- Molecular Weight: 116.16
- MDL number: MFCD00008319
- EINECS: 211-173-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
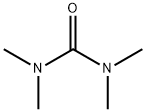
What is 1,1,3,3-Tetramethylurea?
Chemical properties
Tetramethylurea is a clear colorless to pale yellow liquid with mild aromatic odor that is miscible with water and many organic solvents. Unusual for an urea is the liquid state of tetramethylurea in a range of > 170 °C.
The Uses of 1,1,3,3-Tetramethylurea
Tetramethylurea is used as a solvent in dyestuff industries, in condensation reaction and intermediates in surfactant. It is utilized for base catalyzed isomerization and alkylation hydrocyanation due to its low permittivity. It reacts with oxalyl chloride to prepare tetramethyl chloroformamidinium chloride, which is used for the conversion of carboxylic acids and dialkyl phosphates to anhydrides and pyrophosphates respectively.
Definition
ChEBI: 1,1,3,3-tetramethylurea is a member of the class of ureas that is urea substituted by methyl groups at positions 1, 1, 3 and 3 respectively. Metabolite observed in cancer metabolism. It has a role as a human metabolite.
Preparation
The reaction of dimethylamine with phosgene in the presence of e. g. 50 % sodium hydroxide solution and subsequent extraction with 1,2-dichloroethane yields tetramethylurea in 95% yield.
Safety Profile
Moderately toxic by ingestion and intravenous routes. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Combustible when exposed to heat, flame, and oxidizers. To fight fire, use foam, mist, spray, dry chemicals. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Dry it over BaO and distil it under nitrogen. It denatures proteins in H2O. [Elbaum & Herskovits Biochemistry 13 1268 1974, Kane Anal Biochem 53 350 1973, Beilstein 4 IV 225.]
Properties of 1,1,3,3-Tetramethylurea
| Melting point: | −1 °C(lit.) |
| Boiling point: | 177 °C(lit.) |
| Density | 0.968 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | 150 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Miscible with water, petroleum ether and common solvents. |
| form | Liquid |
| pka | 2.0(at 25℃) |
| color | Clear colorless to pale yellow |
| Relative polarity | 5 |
| Odor | faint pleasant odor |
| PH | 5-8 (25℃, 1M in H2O) |
| Water Solubility | H2O: 1 M at 20 °C, miscible |
| Merck | 14,9230 |
| BRN | 773898 |
| Dielectric constant | 24.460000000000001 |
| CAS DataBase Reference | 632-22-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Urea, tetramethyl-(632-22-4) |
| EPA Substance Registry System | Tetramethylurea (632-22-4) |
Safety information for 1,1,3,3-Tetramethylurea
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,1,3,3-Tetramethylurea
| InChIKey | AVQQQNCBBIEMEU-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Tetramethylurea, 99% CAS 632-22-4View Details
Tetramethylurea, 99% CAS 632-22-4View Details
632-22-4 -
 Tetramethylurea CAS 632-22-4View Details
Tetramethylurea CAS 632-22-4View Details
632-22-4 -
 Tetramethylurea CAS 632-22-4View Details
Tetramethylurea CAS 632-22-4View Details
632-22-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8