1,1-DICHLOROPROPENE
Synonym(s):1,1-Dichloro-1-propene
- CAS NO.:563-58-6
- Empirical Formula: C3H4Cl2
- Molecular Weight: 110.97
- MDL number: MFCD00000839
- EINECS: 209-253-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
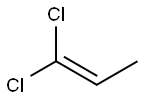
What is 1,1-DICHLOROPROPENE?
Description
1,3-Dichloropropene is a colorless to strawcolored liquid with a sharp, sweet, irritating, chloroformlike odor. Molecular weight=110.97; Specific gravity(H2O:1)=1.21; Boiling point=107.7℃; 103-110℃(mixed cis- and trans-isomers); Freezing/Meltingpoint=283.8℃; Vapor pressure=29 mmHg at 20℃;Flash point=35℃. The explosive limits are LEL=5.3%;UEL=14.5%. Hazard Identification (based on NFPA-704M Rating System): Health 2, Flammability 3, Reactivity 0.Practically insoluble in water; solubility=0.2%.
Chemical properties
1,3-Dichloropropene is a colorless to strawcolored liquid. Sharp, sweet, irritating, chloroform-like odor.
The Uses of 1,1-DICHLOROPROPENE
1,1-Dichloropropene is useful in the preparation of novel 1,1-dichloropropene derivatives with insecticidal activity against Prodenia litura for Agricultural purposes.
Potential Exposure
Used as a soil fumigant prior to planting crops, such as cotton, sugar beet, potatoes; used in combinations with dichloropropanes as a soil fumigant. Workers engaged in manufacture, formulation and application of this soil fumigant and nematocide.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with 1,3-dichloropropene you should betrained on its proper handling and storage. Before enteringconfined space where this chemical may be present, checkto make sure that an explosive concentration does not exist.1,3-Dichloropropene must be stored to avoid contact withaluminum or magnesium compounds, substances containingfluorine, chlorine, bromine or iodine, and alkaline or corrosive materials, since violent reactions occur. Store in tightlyclosed containers in a cool, well-ventilated area away fromheat. Separate outside storage is preferred. Sources of ignition, such as smoking and open flames are prohibited where1,3-dichloropropene is handled, used, or stored. Metal containers involving the transfer of=gallons or more of 1,3-dichloropropene should be grounded and bonded. Drumsmust be equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof 1,3-dichloropropene. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045.
Shipping
UN2047 Dichloropropene, Hazard Class: 3; Labels: 3-Flammable liquid.
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with strong acids; oxidizers, aluminum or magnesium compounds; aliphatic amines; alkanolamines, alkaline materials; halogens, or corrosives. Note: Epichlorohydrin may be added as a stabilizer.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 1,1-DICHLOROPROPENE
| Boiling point: | 76.55°C |
| Density | 1.1864 |
| refractive index | 1.4450 |
| Flash point: | 11°C |
| storage temp. | Refrigerator |
| BRN | 1734809 |
| CAS DataBase Reference | 563-58-6(CAS DataBase Reference) |
| EPA Substance Registry System | 1,1-Dichloropropene (563-58-6) |
Safety information for 1,1-DICHLOROPROPENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1,1-DICHLOROPROPENE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N1-[1-(4-CHLOROPHENYL)-1-METHYL-1-OXO-LAMBDA6-SULFANYLIDENE]-2,3,3-TRICHLOROACRYLAMIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB4114523.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1