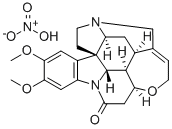
10,11-Dimethoxystrychnine
Synonym(s):(-)-Brucine;2,3-Dimethoxy strychnine
- CAS NO.:357-57-3
- Empirical Formula: C23H26N2O4
- Molecular Weight: 394.46
- MDL number: MFCD00005942
- EINECS: 206-614-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
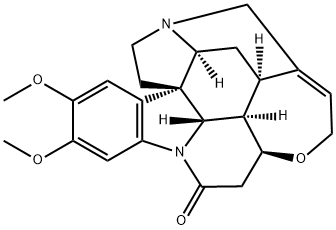
What is 10,11-Dimethoxystrychnine?
Description
As a Chinese medicine, brucine was firstly recorded in Compendium of Materia Medica. The Chinese name for brucine is “Maqian” because it looks like ancient coins with horse icon, where “Ma” refers to “horse” and “qian” refers to “coins.” This traditional Chinese herb has cold, bitter, and toxic properties. It can be used to treat typhoid fever, throat pain, and lumps. Brucine is mainly extracted from the traditional Chinese medicine “Maqian.” In 1819, Pelletier and Caventou firstly isolated the alkaline brucine from the seeds and bark of Strychnos nux-vomica L.?Brucine is the dry and mature seeds of Loganiaceae Evergreen tree plants including Strychnos nux-vomica L. and Strychnos pierriana A.W. Hill.
Chemical properties
White powder
Physical properties
Appearance: white crystalline powder; tastes very bitter. Solubility: slightly soluble in water; soluble in ether, chloroform, ethanol, methanol, and other organic solvents . Melting point: 175–178?°C. Specific optical rotation: ?118°.
The Uses of 10,11-Dimethoxystrychnine
10,11-Dimethoxystrychnine can be used for antineoplastic, convulsant.
The Uses of 10,11-Dimethoxystrychnine
Brucine occurs in the seeds of strychnosspecies (Strychnos nux vomica L. and otherspecies). It is used for denaturing alcoholsand oils; for separating racemic mixtures;and as an additive to lubricants. It is alsoused for colorimetric analysis of nitrate.Therapeutically, it is used as a stimulant.
The Uses of 10,11-Dimethoxystrychnine
Brucine is used as a denaturing alcohol, lubricant additive, separation of racemic mixtures. Proposed to reduce inflammatory mediators including COX-2, PGE2 and thromboxane B2. Able to induce cell apoptosis, with cytotoxic and antiproliferative effects.
Definition
ChEBI: Brucine is a monoterpenoid indole alkaloid and an organic heteroheptacyclic compound.
General Description
A white crystalline solid. Combustible but may require some effort to ignite. Toxic by inhalation (vapor, dust, etc.) and ingestion.
Air & Water Reactions
Slightly soluble in water.
Hazard
Poison by ingestion and inhalation.
Health Hazard
Chemical is toxic if inhaled, swallowed, or absorbed through skin. Inhalation produces intense bitter taste. Ingestion causes nausea, vomiting, restlessness, excitement, twitching, and (rarely) convulsions. Contact with dust irritates eyes.
Health Hazard
The toxicity of brucine is similar to that ofstrychnine. It is a very poisonous alkaloid. Itis a strong convulsant; excites all portions ofthe central nervous system. Toxic symptomsinclude headache, tremor, muscular rigidity,and convulsions. Death may occur fromrespiratory arrest after a few convulsions.
LD50 value, subcutaneous (mice): 60 mg/kg.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fires.
Agricultural Uses
Rodenticide: Used in the manufacture of other chemicals, in perfumes, as a medication for animals, and as a poison for rodents
Trade name
DOLCO MOUSE CEREAL®; PIED PIPER MOUSE SEED® (Brucine)
Pharmacology
1. Analgesic effects: Brucine is reported to exert analgesic effects through both
central and peripheral pathways. Possible mechanisms of the analgesic effects
include blocking the voltage-gated sodium channel, inhibiting the synthesis of
PGE2, acting on the adrenergic receptor and the L-arginine-NO pathway. Brucine
(0.48?mg/kg) can significantly elevate the pain threshold in mice.
2. Anti-tumor effects: Studies have shown that brucine inhibits the cell proliferation in breast cancer, liver cancer, leukemia, and Ehrlich ascites tumors by inducing tumor cell apoptosis, inhibiting angiogenesis, reversing tumor multidrug
resistance, and regulating the expression of various cytokines. In addition, at the
dose of 320?μg/ml, the in?vitro hepatocarcinoma cell growth inhibition rate of
brucine is nearly 100%.
3. Anti-pathogen effects: In vitro study shows that 0.1% brucine can completely
inhibit the growth of Haemophilus influenzae, Streptococcus pneumoniae, and
alpha Streptococcus and Mlicrococcus catarrhalis. At the dose of 500?μg/ml, the
inhibitory rate of brucine on human immunodeficiency virus reverse transcriptase is above 30%.
4. Other pharmacological effects: Anti-inflammation, regulation of the immune
system, regulation of the cardiovascular system, antitussive, expectorant, and
antiasthenic and antibacterial effects.
Clinical Use
The applications of brucine include reducing swelling; resolving mass; activating
meridians to stop pain; attenuating rheumatism, stubborn paralysis, and numbness
paralysis; alleviating bruises; etc. It has been used for nearly a thousand years and
achieved prominent clinical efficacy. In the clinical practice, brucine is used as a
compatibility prescription or compound medicine in treating rheumatism, rheumatoid arthritis, stroke hemiplegia, dementia, retinopathy, and orthopedic and other
surgical diseases.
Brucine is the main active ingredient in Strychnos and plays an important role in
treating diseases. However, brucine is also a toxic ingredient, which limits the scope
of its clinical application. Further studies of its pharmacological effects and toxicity
will improve its clinical application.
Safety Profile
A poison by subcutaneous, intravenous, and intraperitoneal routes. An alkaloid-like strychnine, but one-sixth as toxic. When heated to decomposition it emits toxic fumes of Nx,. See also STRYCHNINE
Potential Exposure
Drug, NaturalProduct. Brucine is used as denaturant for Ethanol, alsoresolving agent for isomer separation; in the manufacture ofother chemicals, in perfumes, as a medication for animals,and as a poison for rodents. It is an alkaloid, produced fromstrychnos seeds.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Protect against physical damage.Outside or detached storage is preferred. Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with brucine you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from strongoxidizers.
Shipping
Label: “POISONOUS/TOXIC MATERIALS.” Itfalls in Hazard Class 6.1 and Packing Group I.
Purification Methods
Crystallise brucine once from water or aqueous Me2CO (as the tetrahydrate), then suspend it in CHCl3 and shake with anhydrous Na2SO4 (to dehydrate the brucine, which then dissolves). Precipitate it by pouring the solution into a large bulk of dry pet ether (b 40-60o), filter and heat to 120o in a high vacuum [Turner J Chem Soc 842 1951]. The tetrahydrate crystallises from a mixture of EtOH and H2O as colourless elongated needles [Eeles Acta Cryst 6 809 1953, Beilstein 27 III/IV 7875.] VERY POISONOUS
Incompatibilities
Reacts with strong oxidizers. Finely dispersed material in air can cause dust explosions.
Properties of 10,11-Dimethoxystrychnine
| Melting point: | 175-178 °C (dec.)(lit.) |
| Boiling point: | 518.67°C (rough estimate) |
| alpha | -118 º (c=2.5 in chloroform) |
| Density | 1.2119 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Store at RT |
| solubility | >15.6mg/mL in DMSO |
| form | Powder |
| pka | 8.28(at 25℃) |
| color | White to light beige |
| Water Solubility | Soluble in alcohol, chloroform, and benzene, slightly soluble in water, ether, and glycerol |
| Merck | 14,1455 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 357-57-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Brucine(357-57-3) |
| EPA Substance Registry System | Brucine (357-57-3) |
Safety information for 10,11-Dimethoxystrychnine
| Signal word | Warning |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H330:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for 10,11-Dimethoxystrychnine
10,11-Dimethoxystrychnine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
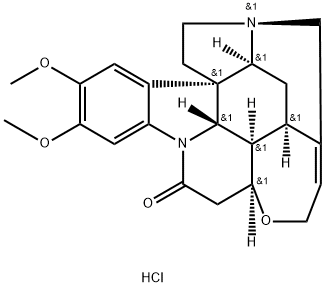
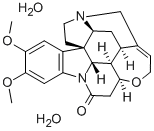



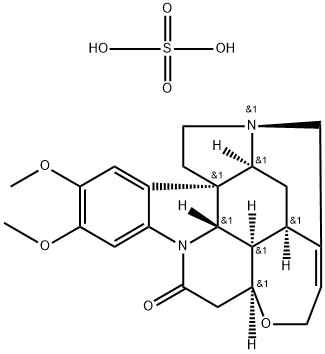

You may like
-
 Brucine, GR 99%+ CAS 357-57-3View Details
Brucine, GR 99%+ CAS 357-57-3View Details
357-57-3 -
 Brucine Anhydrous CAS 357-57-3View Details
Brucine Anhydrous CAS 357-57-3View Details
357-57-3 -
 Brucine 98% CAS 357-57-3View Details
Brucine 98% CAS 357-57-3View Details
357-57-3 -
 BRUCINE ANHYDROUS AR CAS 357-57-3View Details
BRUCINE ANHYDROUS AR CAS 357-57-3View Details
357-57-3 -
 Brucine anhydrous 99% AR CAS 357-57-3View Details
Brucine anhydrous 99% AR CAS 357-57-3View Details
357-57-3 -
 (-)-Brucine CAS 357-57-3View Details
(-)-Brucine CAS 357-57-3View Details
357-57-3 -
 Brucine CAS 357-57-3View Details
Brucine CAS 357-57-3View Details
357-57-3 -
 Brucine CAS 357-57-3View Details
Brucine CAS 357-57-3View Details
357-57-3
