1-METHYLPHENANTHRENE
- CAS NO.:832-69-9
- Empirical Formula: C15H12
- Molecular Weight: 192.26
- MDL number: MFCD00058942
- EINECS: 212-622-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:12:45
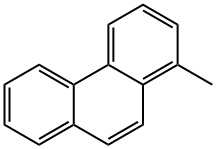
What is 1-METHYLPHENANTHRENE?
The Uses of 1-METHYLPHENANTHRENE
1-Methylphenanthrene is a polycyclic aromatic hydrocarbon that has been found in particulate matter from small-scale biomass combustion from old and modern technologies and that has caused acute systemic and lung inflammation in mice after intratracheal aspiration.
Definition
ChEBI: A member of the class of phenanthrenes that is phenanthrene substituted by a methyl group at position 1.
Synthesis Reference(s)
The Journal of Organic Chemistry, 45, p. 2009, 1980 DOI: 10.1021/jo01298a054
Tetrahedron Letters, 6, p. 359, 1965
General Description
White crystalline powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as 1-METHYLPHENANTHRENE, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction. 1-METHYLPHENANTHRENE is sensitive to excessive heat and light.
Fire Hazard
Flash point data for 1-METHYLPHENANTHRENE are not available. 1-METHYLPHENANTHRENE is probably combustible.
Source
Detected in 8 diesel fuels at concentrations ranging from 0.10 to 210 mg/L with a mean
value of 44.33 mg/L (Westerholm and Li, 1994). Identified in a South Louisiana crude oil at a
concentration of 111 ppm (Pancirov and Brown, 1975). Schauer et al. (1999) reported 1-
methylphenanthrene in diesel fuel at a concentration of 28 μg/g and in a diesel-powered mediumduty
truck exhaust at an emission rate of 17.0 μg/km.
California Phase II reformulated gasoline contained 1-methylphenathrene at a concentration of
3.91 g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were approximately 1.63 and 122 μg/km, respectively (Schauer et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The respective gas-phase
and particle-phase emission rates of 1-methylphenanthrene were 2.22 and 0.579 mg/kg of pine
burned and 1.04 and 0.050 mg/kg of oak burned. The gas-phase emission rate was 0.720 mg/kg of
eucalyptus burned.
Properties of 1-METHYLPHENANTHRENE
| Melting point: | 123°C |
| Boiling point: | 353.25°C (rough estimate) |
| Density | 1.0561 (estimate) |
| refractive index | 1.6031 (estimate) |
| storage temp. | Room Temperature |
| solubility | Soluble in alcohol (Weast, 1986) |
| form | Solid |
| color | White powder or solid. |
| Water Solubility | 269ug/L(25 ºC) |
| Henry's Law Constant | 1.56, 2.33, 3.42, 4.93, and 6.68 x 10-5 atm?m3/mol at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al.,
1998) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| EPA Substance Registry System | 1-Methylphenanthrene (832-69-9) |
Safety information for 1-METHYLPHENANTHRENE
Computed Descriptors for 1-METHYLPHENANTHRENE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![9,10-DIHYDROBENZO[A]PYREN-7(8H)-ONE](https://img.chemicalbook.in/CAS/GIF/3331-46-2.gif)
![3-METHYLPHENANTHRO[3,4-C]PHENANTHRENE](https://img.chemicalbook.in/CAS/GIF/83844-21-7.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4