1-Hexene
- CAS NO.:592-41-6
- Empirical Formula: C6H12
- Molecular Weight: 84.16
- MDL number: MFCD00009505
- EINECS: 209-753-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:34
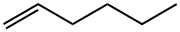
What is 1-Hexene?
Description
Hexene is a colorless liquid. Molecu larweight= 84.14;Boiling point= 63-65℃;Freezing/Melting point=一140℃; Flash point= - 7℃; Autoignitiontemperature = 253℃. Explosive Limits:LEL= 1.2%;UEL= 6.9%. Hazard Identification (based on NFPA-704 MRating System): Health 1, Flammability 3, Reactivity 0.Insoluble in water.
Chemical properties
colourless liquid
Chemical properties
1-Hexene, C6H12, is a colorless liquid, which is highly volatile and flammable. Hexene is a component of refinery gas and coffee aroma.
Physical properties
Clear, colorless, flammable liquid with a characteristic, sweetish odor similar to hexane or 1-pentene. An odor threshold concentration of 140 ppbv was reported by Nagata and Takeuchi (1990).
The Uses of 1-Hexene
1-Hexene is used as a solvent, paint thinner and a medium for conducting chemical reactions. It is a commoner and used in the preparation of both low density and high density polyethylenes. It acts as an adhesives, lubricants, lubricant additives and sealant chemicals. Further, it is used in the preparation of hex-1-en-3-one, heptanal and heptanoic acid. It is also employed in the synthesis of linear plasticizers, oxo-alcohols, motor fuels, automotive additives and biodegradable surfactants. In addition to this, it is used in the preparation of mercaptans, flavors and fragrances, alkyl metals, halides and alkyl silanes.
The Uses of 1-Hexene
The Uses of 1-Hexene
1-Hexene is primarily used in the synthesis of poly(1-hexene) and a variety of polyethylene copolymers.
What are the applications of Application
1-Hexene is a useful hexene for proteomics research
Definition
ChEBI: An alkene that is hexane carrying a double bond at position 1.
General Description
A clear colorless liquid with a petroleum like odor. Flash point -9°F. Less dense than water and insoluble in water. Vapors heavier than air. Used as a solvent, paint thinner, and chemical reaction medium.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1--HEXENE may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas.
Hazard
Irritant. Highly flammable, dangerous fire risk.
Health Hazard
Inhalation may cause giddiness or incoordination similar to that from gasoline vapor. Prolonged exposure to high concentrations may induce loss of consciousness or death.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Potential Exposure
Those involved in its use in organicsynthesis. .Used in fuels,and to make flavors,perfumes,dyes, and plastic resins.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled,remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
California Phase II reformulated gasoline contained 1-hexene at a concentration of 770
mg/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic converters were 430 and 18,400 μg/km, respectively (Schauer et al., 2002).
According to Chevron Phillips Company’s (2005) product literature, the maximum
concentration present in 99.7% tert-butyl mercaptan is 0.05 wt %.
Environmental Fate
Biological. Biooxidation of 1-hexene may occur yielding 5-hexen-1-ol, which may oxidize to
give 5-hexenoic acid (Dugan, 1972). Washed cell suspensions of bacteria belonging to the genera
Mycobacterium, Nocardia, Xanthobacter, and Pseudomonas and growing on selected alkenes
metabolized 1-hexene to 1,2-epoxyhexane (Van Ginkel et al., 1987).
Photolytic. The following rate constants were reported for the reaction of 1-hexene and OH
radicals in the atmosphere: 1.9 x 10-12 cm3/molecule?sec at 300 K (Hendry and Kenley, 1979); 3.75
x 10-11 cm3/molecule?sec at 295 K (Atkinson and Carter, 1984); 3.18 x 10-11 cm3/molecule?sec
(Atkinson, 1990). The following rate constants were reported for the reaction of 1-hexene and
ozone in the atmosphere: 1.10 x 10-17 cm3/molecule?sec (Bufalini and Altshuller, 1985); 9.0 x 10-17
cm3/molecule?sec (Cadle and Schadt, 1952); 1.40 x 10-17 cm3/molecule?sec (Cox and Penkett,
1972); 1.08 x 10-17 cm3/molecule?sec at 294 K (Adeniji et al., 1981).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water.
1-Hexene is not expected to hydroxyze in water.
Storage
Color Code- -Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with hexene you should be trained on itsproper handling and storage. Before entering confined spacewhere hexene may be present, check to make sure that anexplosive concentration does not exist. Store in tightlyclosed containers in a cool, well-ventilated area away fromoxidizers and strong acids. Metal containers involving thetransfer of this chemical should be grounded and bonded.Where possible, automatically pump liquid from drums orother storage containers to process containers. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof this chemical. Sources of ignition, such as smoking andopen flames, are prohibited where this chemical is used,handled, or stored in a manner that could create a potentialfire or explosion hazard. Wherever this chemical is used,handled, manufactured, or stored, use explosion-proof elec-trical equipment and fittings.
Shipping
This compound requiresa shipping label of“FL .AMMABLE LIQUID." It falls in Hazard Class 3;andPacking Group II.
Purification Methods
Purify it by stirring over Na/K alloy for at least 6hours, then fractionally distil it from sodium under nitrogen. [Beilstein 1 IV 828.]
Incompatibilities
Formsexplosivemixturewithair.Violent reaction with oxidizers, strong acids.
Properties of 1-Hexene
| Melting point: | -140 °C |
| Boiling point: | 60-66 °C (lit.) |
| Density | 0.678 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 155 mm Hg ( 21.1 °C) |
| refractive index | n |
| Flash point: | −19 °F |
| storage temp. | Refrigerator |
| solubility | Soluble in alcohol, benzene, chloroform, ether, petroleum (Weast, 1986), and many other
hydrocarbons including alkenes amd alkanes. |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| Specific Gravity | 0.673 |
| color | Clear colorless |
| Odor | Mild, pleasant. |
| Odor Threshold | 0.14ppm |
| Water Solubility | 0.005 g/100 mL |
| BRN | 1209240 |
| Henry's Law Constant | (atm?m3/mol):
0.435 at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | ACGIH TLV: TWA 30 ppm (adopted). |
| Dielectric constant | 2.1(Ambient) |
| Stability: | Stable. Highly flammable - note low flash point. Incompatible with strong oxidizing agents, strong acids, combustible material. |
| CAS DataBase Reference | 592-41-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Hexene(592-41-6) |
| EPA Substance Registry System | 1-Hexene (592-41-6) |
Safety information for 1-Hexene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 1-Hexene
| InChIKey | LIKMAJRDDDTEIG-UHFFFAOYSA-N |
1-Hexene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 1-Hexene, 97% 592-41-6 99%View Details
1-Hexene, 97% 592-41-6 99%View Details
592-41-6 -
![1-Hexene [Standard Material for GC] CAS 592-41-6](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-Hexene [Standard Material for GC] CAS 592-41-6View Details
1-Hexene [Standard Material for GC] CAS 592-41-6View Details
592-41-6 -
 1-Hexene CAS 592-41-6View Details
1-Hexene CAS 592-41-6View Details
592-41-6 -
 1-Hexene CAS 592-41-6View Details
1-Hexene CAS 592-41-6View Details
592-41-6 -
 1-Hexene CAS 592-41-6View Details
1-Hexene CAS 592-41-6View Details
592-41-6 -
 1-Hexene CAS 592-41-6View Details
1-Hexene CAS 592-41-6View Details
592-41-6 -
 Liquid 1-Hexene (592-41-6), Technical Grade, 25 LitreView Details
Liquid 1-Hexene (592-41-6), Technical Grade, 25 LitreView Details
592-41-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
