1-Chlorobutane
Synonym(s):1-Chlorobutane;Butyl Chloride;n-Butyl chloride
- CAS NO.:109-69-3
- Empirical Formula: C4H9Cl
- Molecular Weight: 92.57
- MDL number: MFCD00001009
- EINECS: 203-696-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-13 14:48:16

What is 1-Chlorobutane?
Description
Butyl chloride is a highly flammable, clear,colorless liquid. Molecular weight = 92.6 (n- and sec-isomers); Boiling point = 77℃; 69℃ (sec-isomer); Specificgravity (H2O:1) = 0.89; Freezing/Melting point =-123℃; Flash point = 9℃; - 10℃ (sec-isomer);Relative vapor density (air =1) = 3.2 (n- and sec-isomers);Vapor pressure = 81 mmHg at 20℃; Autoignitiontemperature = 240℃. Explosive limits: LEL = 1.8%;UEL = 10.1%. Insoluble in water. Hazard Identification(based on NFPA-704 M Rating System): Health 2,Flammability 3, Reactivity 0; (sec-) Health 2, Flammability4, Reactivity 0. Practically insoluble in water;solubility = 0.7% at 12℃.
Chemical properties
1-Chlorobutane is a highly flammable, clear, colorless liquid at standard temperature and pressure. The density is 0.886 g/cm3, which is lower than that of water. It does not react with water, is classified as highly flammable, but it is neither an oxidizer nor an explosive. However, vapors can form explosive mixtures with air. The substance self-ignites at 245℃.
The Uses of 1-Chlorobutane
1-Chlorobutane is used as an intermediate for the production of other chemicals in the chemical industry.
1-chlorobutane is a common extraction solvent in the forensic toxicology arena. A benefit of 1-chlorobutane is that it is less dense than water and therefore settles above the aqueous layer.
As butylating agent in organic synthesis, e.g., in the manufacture of butyl cellulose.
1-chlorobutane will be halogenated to produce dichlorobutane using sulfuryl chloride via a free-radical chain reaction mechanism.
The Uses of 1-Chlorobutane
1-Chlorobutane is used as intermediates and solvents. It is used for HPLC, spectrophotometry and environmental testing. It is used as alkylating reagent in organic and organometallic compound synthesis. It is also employed as an antihelmintic in veterinary medicine.
The Uses of 1-Chlorobutane
1-Chlorobutane may be used in the synthesis of ionic liquids, 1-butyl-3-methylimidazolium hydrogen sulfate ([Bmim]+[HSO4]-) and 1-butyl-3-methylimidazolium dihydrogen phosphate ([Bmim]+[H2PO4]-).
What are the applications of Application
1-Chlorobutane is a chromatography solvent
Preparation
1-Chlorobutane is obtained by esterification of n-butanol with hydrogen chloride or hydrochloric acid at 100℃ either without a catalyst or utilizing the accelerating effect of zinc chloride, tripentylamine hydrochloride, or phosphorus pentachloride. n-Butyl chloride is also obtained, along with 2-chlorobutane, by the chlorination of butane over aluminum oxide at 200℃.
Synthesis Reference(s)
Journal of the American Chemical Society, 87, p. 2500, 1965 DOI: 10.1021/ja01089a041
The Journal of Organic Chemistry, 59, p. 4687, 1994 DOI: 10.1021/jo00095a054
General Description
A water white liquid with a sharp odor. Flash point 20°F. Boiling point 77-78°C (173°F). Density 7.5 lb / gal. Slightly soluble in water. Vapors are heavier than air. Used in the manufacture of a variety of organic chemicals.
Air & Water Reactions
Highly flammable. May react with atmospheric moisture over prolonged periods of exposure. Slightly soluble in water.
Reactivity Profile
1-Chlorobutane is incompatible with oxidizing agents and strong bases. Reacts with aluminum powder, liquid oxygen, potassium and sodium . Emits phosgene gas when heated to decomposition,. Reacts with aluminum and magnesium. May be sensitive to heat.
Hazard
Toxic on prolonged inhalation. Flammable, dangerous fire risk.
Health Hazard
1-Chlorobutane has a low acute toxicity by oral exposure. mildly irritating to the skin and eyes, liquid may cause rash due to removal of skin oils. Ingestion or skin absorbtion may cause intestinal upset, cramping, and central nervous system depression. 1- Chlorobutane is also not considered mutagenic, carcinogenic or toxic to reproduction.
Fire Hazard
Special Hazards of Combustion Products: May produce phosgene gas in fire
Flammability and Explosibility
Flammable
Safety Profile
Moderately toxic by ingestion. Mutation data reported. See CHLORINATED HYDROCARBONS, ALIPHATIC. Skin and eye irritant. Dangerous fire hazard when exposed to heat or flame. Moderately explosive when exposed to flame. When heated to decomposition it emits hghly toxic fumes of phosgene and Cl-. To fight fire, use foam, COa, dry chemical. Incompatible with oxidizing materials
Potential Exposure
Butyl chloride is used as a solvent; as a medicine to control worms, and to make other chemicals
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. stopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Butyl chloride is incompatible with oxidizers (such as perchlorates, peroxides, permanganates, chlorates and nitrates).Store in tightly closed containers in a cool, well-ventilatedarea. Sources of ignition, such as smoking and open flames,are prohibited where Butyl chloride is handled, used, orstored. Metal containers involving the transfer of 5 gallonsor more of Butyl chloride should be grounded and bonded.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of Butyl chloride.
Shipping
UN1127 Chlorobutanes require, Hazard Class: 3; Labels: 3—Flammable liquid
Purification Methods
Shake it repeatedly with conc H2SO4 (until no further colour develops in the acid), then wash it with water, aqueous NaHCO3 or Na2CO3, and more water. Dry it with CaCl2, or MgSO4 (then with P2O5 if desired), decant and fractionally distil it. Alternatively, a stream of oxygen continuing ca three times as long as is necessary to obtain the first coloration of starch iodide paper by the exit gas. After washing with NaHCO3 solution to hydrolyse ozonides and to remove the resulting organic acid, the liquid is dried and distilled [Chien & Willard J Am Chem Soc 75 6160 1953]. [Beilstein 1 IV 246.]
Incompatibilities
Vapor may form explosive mixture with air. May accumulate static electrical charges, and may cause ignition of its vapors. Water contact slowly forms hydrochloric acid. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alkaline earth, and alkali metals; finely divided metal. Attacks metals in presence of moisture. Attacks some plastics, rubber, or coatings
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of 1-Chlorobutane
| Melting point: | -123 °C (lit.) |
| Boiling point: | 77-78 °C (lit.) |
| Density | 0.886 g/mL at 25 °C (lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 80.1 mm Hg ( 78.4 °C) |
| refractive index | n |
| Flash point: | -12 °F |
| storage temp. | 2-8°C |
| solubility | water: soluble0.11g/L at 20°C |
| form | Viscous Liquid |
| color | White to pink to light brown |
| Odor | Unpleasant odor |
| explosive limit | 1.8-10.1%(V) |
| Water Solubility | 0.5 g/L (20 ºC) |
| Merck | 14,1560 |
| BRN | 1730909 |
| Dielectric constant | 9.6(20℃) |
| Stability: | Stable. Highly flammable. Note low flash point and wide explosion limit range. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 109-69-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Butane, 1-chloro-(109-69-3) |
| EPA Substance Registry System | 1-Chlorobutane (109-69-3) |
Safety information for 1-Chlorobutane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 1-Chlorobutane
| InChIKey | VFWCMGCRMGJXDK-UHFFFAOYSA-N |
1-Chlorobutane manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

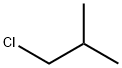
You may like
-
 1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
109-69-3 -
 1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
109-69-3 -
 1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
109-69-3 -
 1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
1-Chlorobutane, Filtered through 0.2μ filters. CAS 109-69-3View Details
109-69-3 -
 n-Butyl Chloride (1-Chlorobutane) for HPLC & UV Spectroscopy CAS 109-69-3View Details
n-Butyl Chloride (1-Chlorobutane) for HPLC & UV Spectroscopy CAS 109-69-3View Details
109-69-3 -
 Butyl chloride, puriss, 99% CAS 109-69-3View Details
Butyl chloride, puriss, 99% CAS 109-69-3View Details
109-69-3 -
 170 kg N Butyl ChlorideView Details
170 kg N Butyl ChlorideView Details
109-69-3 -
 Liquid N-Butyl ChlorideView Details
Liquid N-Butyl ChlorideView Details
109-69-3
